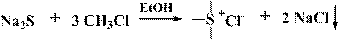Preparation method of trimethyl sulfonium chloride
A technology of trimethylsulfonium chloride and methyl chloride, which is applied in the direction of organic chemistry, can solve the problems of low boiling point of dimethyl sulfide, large environmental impact, toxicity, etc., and achieve short reaction time, low production cost, and high product yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 12.0 g of sodium sulfide nonahydrate and 30 mL of anhydrous methanol into a 50 mL tetrafluoroethylene-lined autoclave, feed 15.2 g of methyl chloride at low temperature, close the autoclave and heat up to an external bath at 65 °C for 10 h, cool, and the reaction solution Filtration, solvent removal by rotary evaporation, 5.4 g of crude product white solids were obtained, and the productive rate was 96.0%. 1 HNMR (D 2 0,600M): 2.87(s,9H). This product was used directly in subsequent reactions.
Embodiment 2
[0018] Add 12.0 g of sodium sulfide nonahydrate and 30 mL of anhydrous methanol into a 50 mL tetrafluoroethylene-lined autoclave, feed 14.7 g of methyl chloride at low temperature, close the autoclave and heat up to an external bath at 90 °C for 6 h, cool, and the reaction solution Filtration, rotary evaporation to remove solvent, almost theoretical yield was obtained as crude product white solid, and this product was recrystallized from ethanol to obtain colorless crystals, 1 HNMR (D 2 0,600M): 2.87(s,9H). The product content measured by nuclear magnetic resonance method is more than 99%.
Embodiment 3
[0020] Add 16.8 g of sodium sulfide nonahydrate and 50 mL of anhydrous n-propanol into a 100 mL tetrafluoroethylene-lined autoclave, feed 17.7 g of methyl chloride at low temperature, close the autoclave and raise the temperature to 100 °C for 4 h, cool, and the reaction solution Filtration, rotary evaporation to remove solvent, crude product white solid 6.6 g, productive rate 83.8%, 1 HNMR (D 2 0,600M): 2.87(s,9H). This product was directly used in the subsequent reaction, and if necessary, it could be recrystallized from ethanol to obtain a higher purity product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com