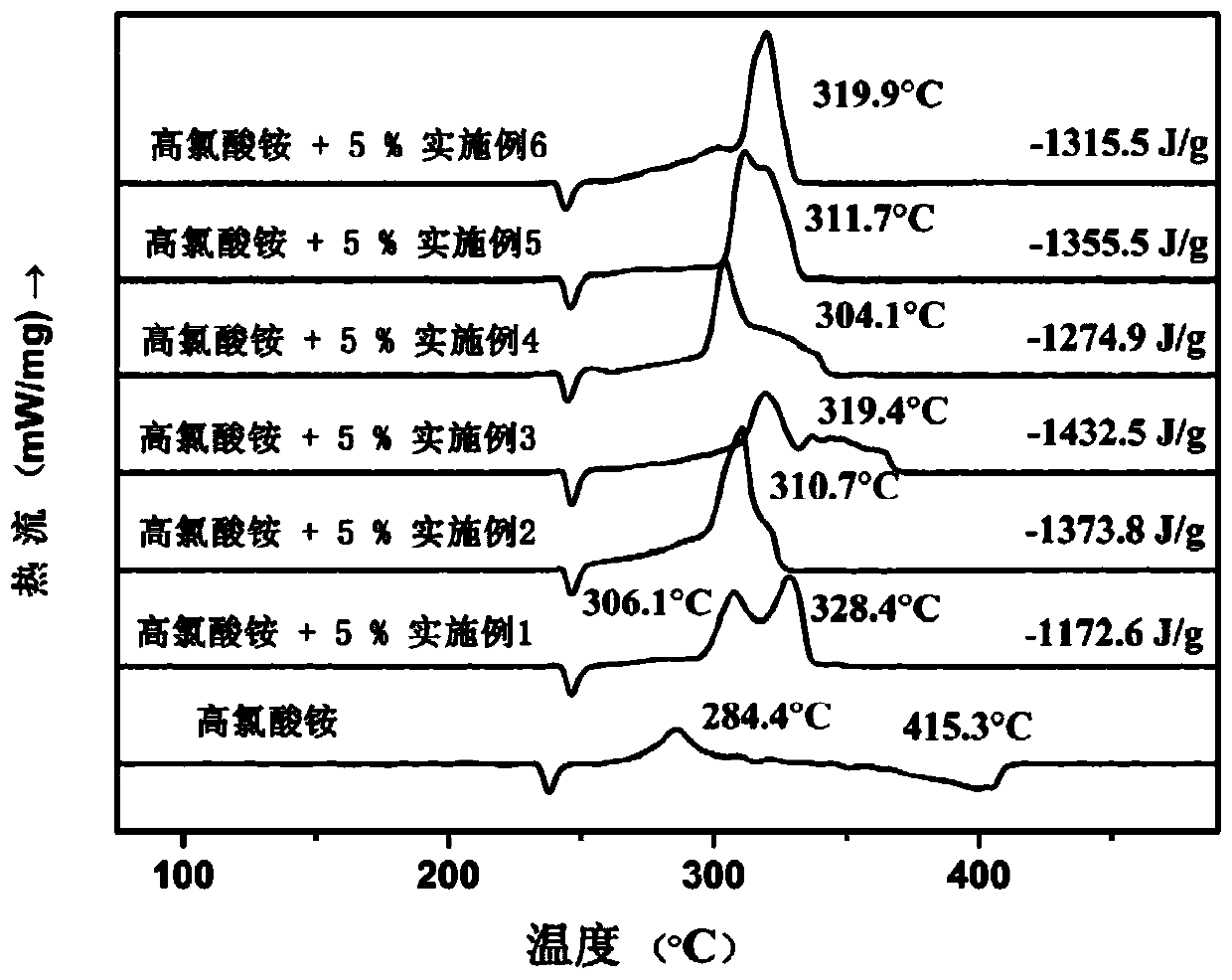
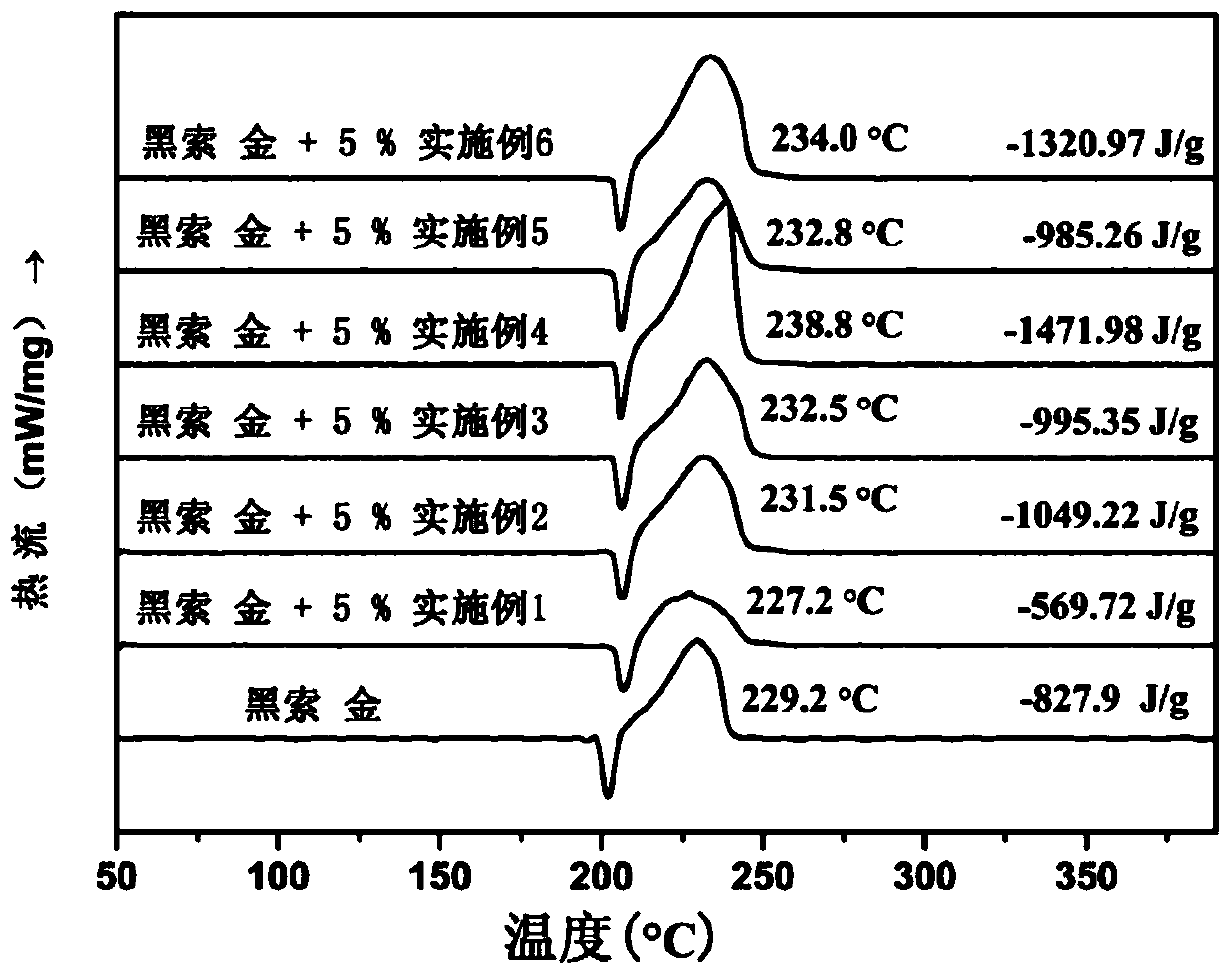
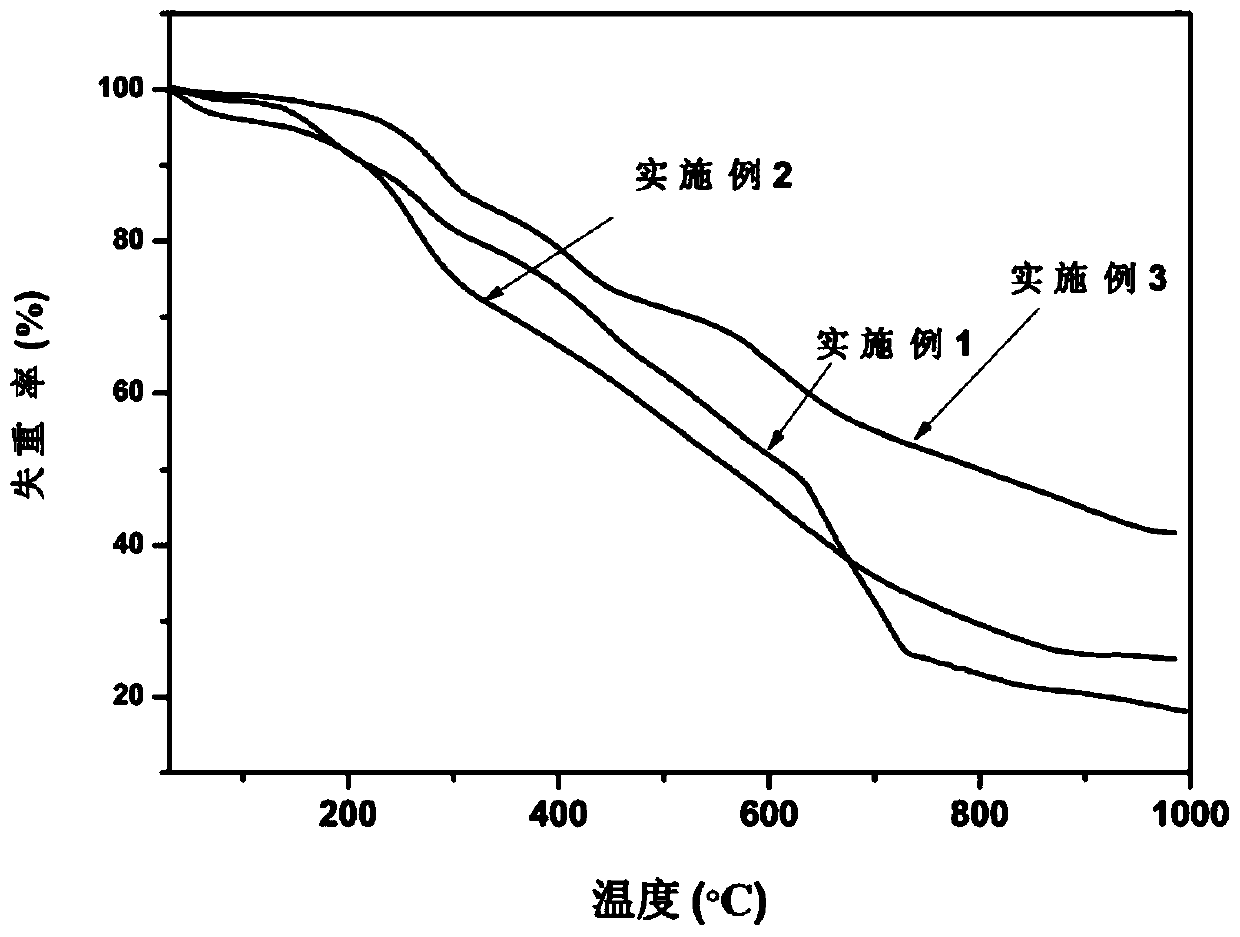
Ferrocene triazole ionic nitrogen-rich energetic metal complex and preparation method thereof
A technology of ferrocene triazole and metal complexes, which is applied in the preparation of organic compounds, metallocenes, organic compounds/hydrides/coordination complex catalysts, etc. problems, to achieve the effects of good thermal stability, low vapor pressure and volatility, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 0.18g (0.5mmol) Zn (ClO 4 ) 2 ·6H 2O was dissolved in a 100mL round-bottomed flask filled with 10mL of distilled water, and when the temperature rose to 60°C, 0.28g (1.0mmol) of ferrocenetriazole Schiff base solution dissolved in 20mL of absolute ethanol was added dropwise to it at the same time and 0.25g (1.0mmol) potassium 1,1,3,3-tetracyanoacrylate solution dissolved in 20mL distilled water, reacted for 3 hours, a brick red precipitate was formed, filtered, and washed the filter cake with absolute ethanol and distilled water, at room temperature Vacuum-dried to obtain the ferrocenetriazole ionic nitrogen-rich energetic metal complex with the following structural formula:
[0031]
[0032] Its yield is 57%, and the structural characterization data is: IR (KBr, cm -1 ):3320(w,br),2194(m),1617(s),1555(vs),1422(m),1280(m),1226(m),1129(s),1093(s),810 (w),641(m),491(m)cm –1 ; Elemental analysis (theoretical calculation value in brackets): C% 48.80 (48.77), H% 2.80 ...
Embodiment 2
[0034] 0.18g (0.5mmol) Cu(ClO 4 ) 2 ·6H 2 O was dissolved in a 100mL round-bottomed flask filled with 10mL of distilled water, and when the temperature rose to 60°C, 0.28g (1.0mmol) of ferrocenetriazole Schiff base solution dissolved in 20mL of absolute ethanol was added dropwise to it at the same time With 0.25g (1.0mmol) sodium picrate solution dissolved in 20mL distilled water, react for 3 hours, a green precipitate is generated, filter, and wash the filter cake with absolute ethanol and distilled water, and dry under vacuum at room temperature to obtain ferrocene with the following structural formula Nitrogen-rich nitrogen-rich energetic metal complexes:
[0035]
[0036] Its yield is 62%, and the structural characterization data is: IR (KBr, cm -1 ):3435(m,br),3081(m),1652(vs),1635(vs),1555(vs),1484(s),1439(m),1324(vs),1174(m),1085 (s),916(w),783(w),703(w),482(m)cm –1 ; Elemental analysis (theoretical calculation value in brackets): C% 43.20 (42.22), H% 2.65 (2.7)...
Embodiment 3
[0038] 0.18g (0.5mmol) Zn (ClO 4 ) 2 ·6H 2 O was dissolved in a 100mL round-bottomed flask filled with 10mL of distilled water, and when the temperature rose to 60°C, 0.28g (1.0mmol) of ferrocenetriazole Schiff base solution dissolved in 20mL of absolute ethanol was added dropwise to it at the same time With 0.25g (1.0mmol) sodium picrate solution dissolved in 20mL distilled water, react for 3 hours, a green precipitate is generated, filter, and wash the filter cake with absolute ethanol and distilled water, and dry under vacuum at room temperature to obtain ferrocene with the following structural formula Nitrogen-rich nitrogen-rich energetic metal complexes:
[0039]
[0040] Its yield is 62%, and the structural characterization data is: IR (KBr, cm -1 ):3329(w,br),3307(w,br),1626(vs),1546(vs),1431(w),1315(m),1271(vs),1085(vs),1022(vs) ,801(s),491(m)cm –1 ; Elemental analysis (theoretical calculation value in brackets): C% 43.20 (42.15), H% 2.87 (2.7), N% 18.32 (18.11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com