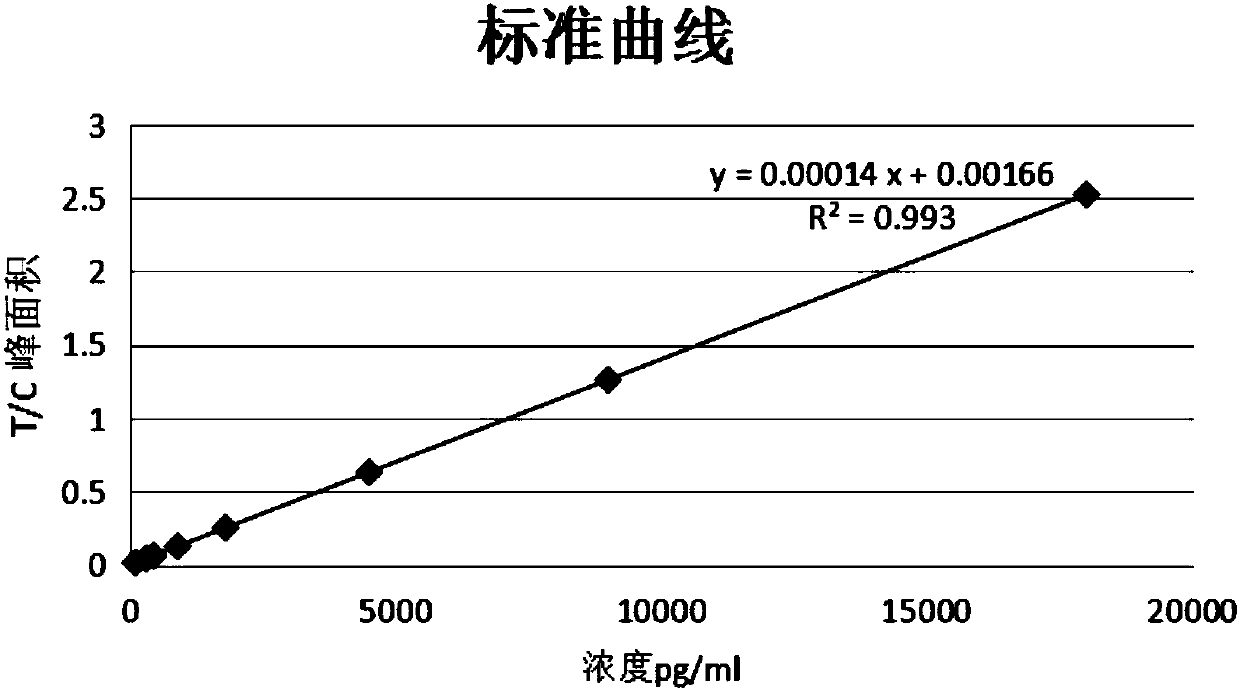
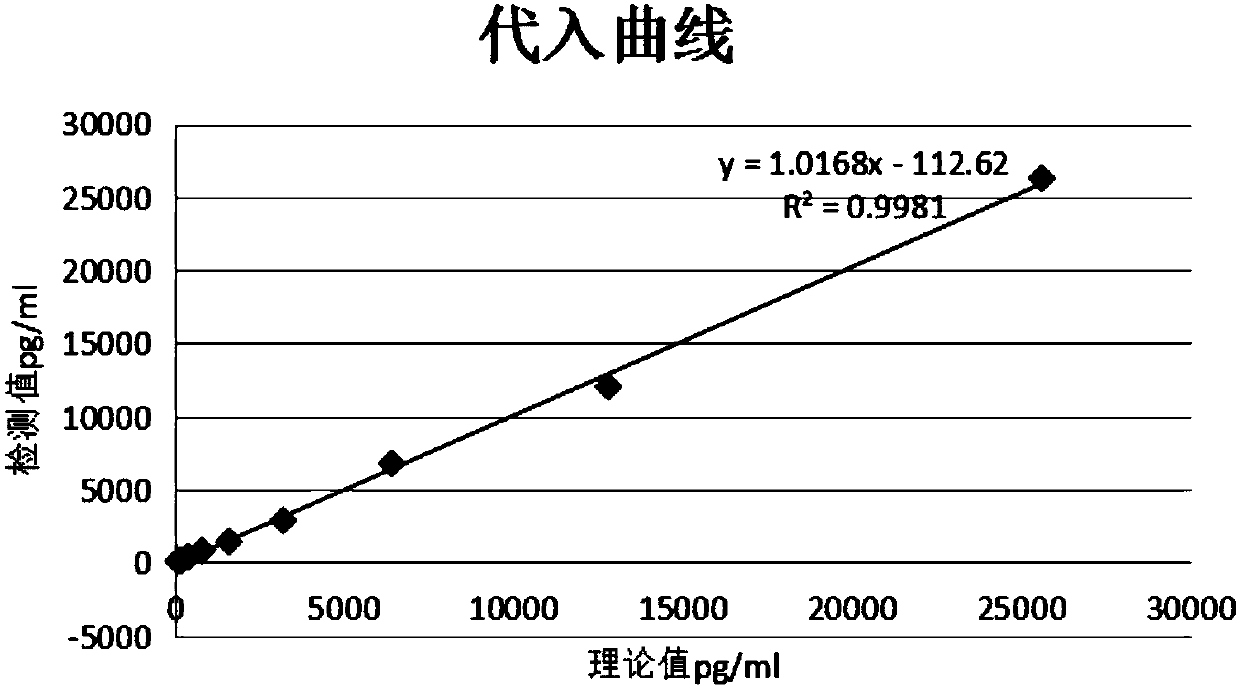
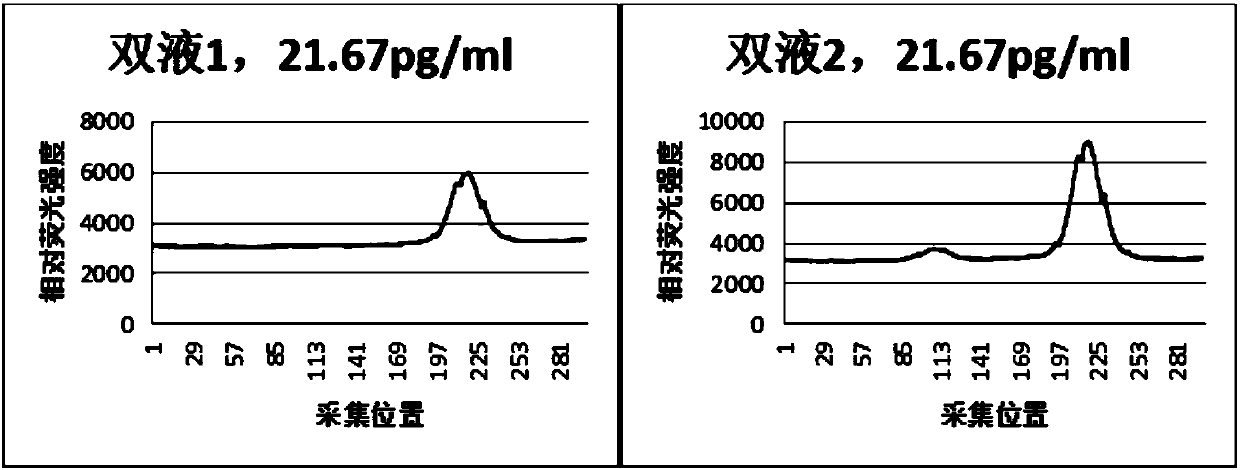
Immunofluorescence quantitative test strip for detecting N-terminal atrial natriuretic peptide
A technology of atrial natriuretic peptide and immunofluorescence, applied in the field of immunology detection, can solve the problems of low automation, poor quantitative accuracy, radioactive contamination, etc., and achieve the effect of simple operation, high accuracy and rapid response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 Storage solution formula and preparation method
[0023] The preferred formulation of the storage solution of the present invention: the mass concentration of PB is 20mM, the percentage concentration of BSA is 1.8%, the percentage concentration of Tween-80 is 0.5%, the percentage concentration of glucose is 0.5%, and the percentage concentration of glycine is 2%, the percentage concentration of PEG4000 is 1%, the percentage concentration of PEG20000 is 1.5%, the percentage concentration of Proclin300 is 0.03%, pH7.8~8.0.
[0024] Preparation method of storage solution: Weigh 0.25g of glucose and dissolve it in 45mL of pure water. Add 1mL of 100mM PB stock solution prepared in advance, shake and mix well. Add 1g of glycine, 0.5g of PEG4000, 0.75g of PEG20000 and 0.9g of BSA successively, shake and mix well; add 0.25mL Tween-80 with a sample gun, mix well repeatedly; add 15μL of Proclin300, shake and mix well, adjust the pH value to 7.8 with 1M HCl ~8.0. F...
Embodiment 2
[0026] Example 2 Combination Pad Pretreatment Liquid Formula and Preparation Method
[0027] The preferred formula of the binding pad pretreatment liquid of the present invention: the percentage concentration of PVA (polyvinyl alcohol) is 2.0%, the percentage concentration of TritonX-100 is 0.8%, the percentage concentration of sucrose is 1.25%, Proclin300 or sodium azide The percentage concentration is 0.03%, PH7.2~7.4.
[0028] Preparation method of bonding pad pretreatment solution: Weigh 10g of PVA and add 480ml of pure water to soak overnight, place at 60°C and heat to dissolve. Add 4mL of Triton X-100 with a sample gun, beat repeatedly; weigh 6.25g of sucrose, put it into a magnetic stirrer to dissolve; add 150μL of Proclin300, adjust the pH value to 7.2-7.4 with 1M HCl, shake and mix. Finally, the volume was adjusted to 500 mL at room temperature, and the filter was sterilized to prepare the binding pad pretreatment solution.
Embodiment 3
[0029] Example 3 N-terminal atrial natriuretic peptide immunofluorescence quantitative test strip
[0030] The preparation process of the N-terminal atrial natriuretic peptide immunofluorescence quantitative test strip is as follows:
[0031](1) Preparation of storage solution: the mass concentration of PB is 20mM, the percentage concentration of BSA is 1.8%, the percentage concentration of Tween-80 is 0.5%, the percentage concentration of glucose is 0.5%, and the percentage concentration of glycine is 2%, the percentage concentration of PEG4000 is 1%, the percentage concentration of PEG20000 is 1.5%, the percentage concentration of Proclin300 is 0.03%, pH7.8~8.0, prepare and mix according to the above formula, filter and sterilize to prepare the storage solution ;
[0032] (2) Preparation of the sample pad: soak the glass fiber membrane with the sample pad treatment solution for 10 minutes, place it in a drying room at 37°C and 30% humidity, and dry it for 5 hours to prepare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com