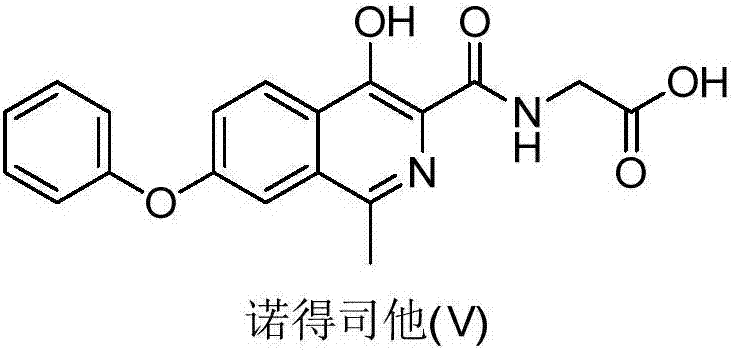
Preparation method of roxadustat
A compound and selected technology, applied in the fields of organic chemistry and medicinal chemistry, can solve the problems of expensive reagent protection and deprotection steps, and achieve the effects of low requirements for reaction equipment, high yield, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
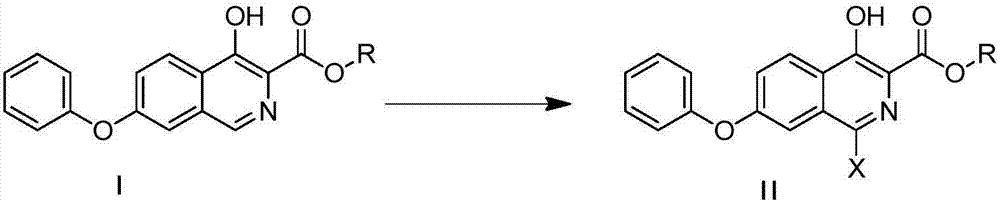
[0037] Compound I generates Compound II
[0038]
[0039]Into a 100mL three-necked flask, add 60mL of methanol and 2.95g of methyl 4-hydroxy-7-phenoxyisoquinoline-3-carboxylate (compound I), lower the stirring temperature to 0-10°C, add 1,3-di Bromo-5,5-dimethylhydantoin 1.57g, slightly exothermic. Heat to reflux for 6h, then lower to 0-10°C, filter with suction, rinse the filter cake with 20mL of methanol, and dry in vacuo to obtain 3.0g of light yellow solid, yield: 81%.
[0040] NMR data ( 1 H NMR, CDCl 3 ,400MHz): δppm 11.793(1H,s,OH),8.398-8.420(1H,d,J=8.8Hz,CH),7.683(1H,s,CH),7.558-7.560(1H,m,CH), 7.532-7.555(1H,m,CH),7.466-7.504(2H,m,CH),7.286-7.312(1H,m,CH),7.160-7.181(2H,d,J=8.4Hz,CH),4.093 (3H,s,CH 3 ).
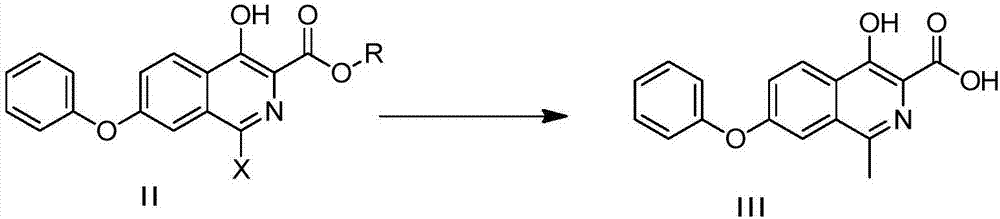
[0041] Compound II generates Compound III
[0042]
[0043] Under the protection of nitrogen, in a 20L three-necked flask, add 6.6L of ethylene glycol methyl ether, 367g of compound II, 176g of methyl boric acid, 766g of tripotassium phosphate, 1326g of...
Embodiment 2
[0054] Compound I generates Compound II
[0055]
[0056] Into a 100mL three-necked flask, add 60mL of methanol and 2.95g of methyl 4-hydroxy-7-phenoxyisoquinoline-3-carboxylate (compound I), lower the stirring temperature to 0-10°C, add 1,3-di Chloro-5,5-dimethylhydantoin 1.1g, slightly exothermic. Heat to reflux for 6h, then lower to 0-10°C, filter with suction, rinse the filter cake with 20mL of methanol, and dry in vacuo to obtain 2.8g of off-white solid, yield: 85%.
[0057] NMR data ( 1 H NMR, CDCl 3 ,400MHz): δppm 11.667(1H,s,OH),8.299-8.322(1H,d,J=9.2Hz,CH),7.744-7.751(1H,d,J=2.8Hz,CH),7.558-7.593( 1H, m, CH), 7.462-7.501 (2H, m, CH), 7.277-7.314 (2H, m, CH), 7.157-7.177 (2H, d, J=8.0Hz, CH), 4.090 (3H, s ,CH 3 ).
[0058] Compound II generates Compound III
[0059]
[0060] Under nitrogen protection, into a 250mL three-necked flask, add DMF 30mL, compound II 3.3g, add sodium iodide 0.3g, methyl boric acid 1.76g, K 2 CO 3 4.1g, 15g of purified water and ...
Embodiment 3
[0071] Compound I generates Compound II
[0072]
[0073] Into a 100mL three-neck flask, add 60mL of dichloromethane and 2.95g of methyl 4-hydroxy-7-phenoxyisoquinoline-3-carboxylate (compound I), lower the stirring temperature to 0-10°C, add N-iodo Substituting succinimide 2.7g, slightly exothermic. Heat to reflux for 7 hours, then lower to 0-10°C, filter with suction, rinse the filter cake with 20 mL of dichloromethane, spin dry, and perform column chromatography (ethyl acetate:petroleum ether=1:10) to obtain 2.0 g of a white solid, Yield 47.5%.
[0074] NMR data ( 1 H NMR, CDCl 3 ,400MHz): δppm 11.772(1H,s,OH),8.347-8.369(1H,d,J=8.8Hz,CH),7.560-7.566(1H,d,J=2.4Hz,CH),7.471-7.524( 3H, m, CH), 7.285-7.296 (1H, m, CH), 7.163-7.185 (2H, m, CH), 4.089 (3H, s, CH 3 ).
[0075] Compound II generates Compound III
[0076]
[0077] Under nitrogen protection, into a 250mL three-necked flask, add DMF 30mL, compound II 4.21g, methyl boric acid 1.76g, K 2 CO 3 4.1g, 15g of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com