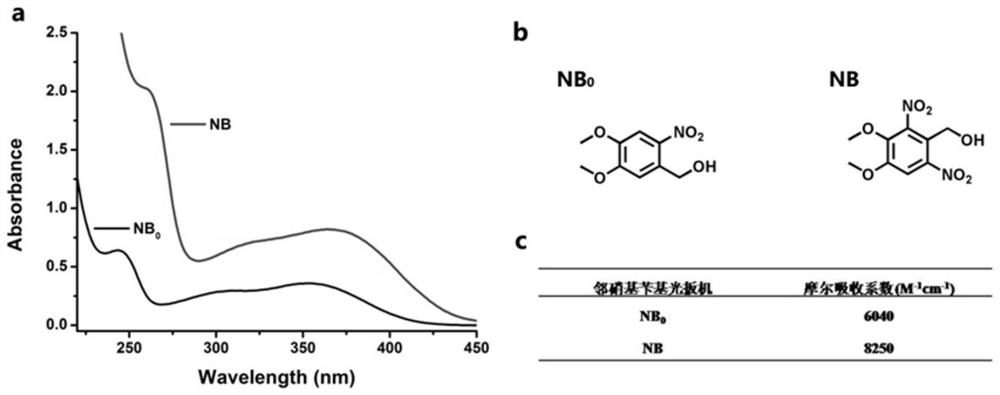
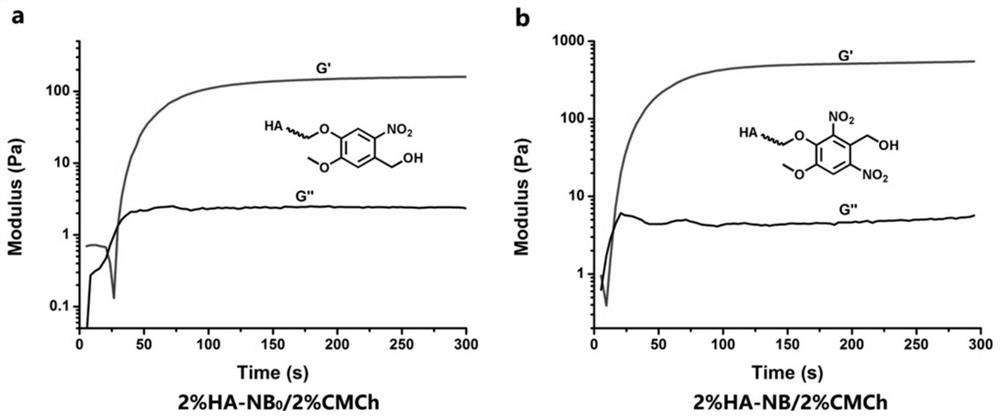
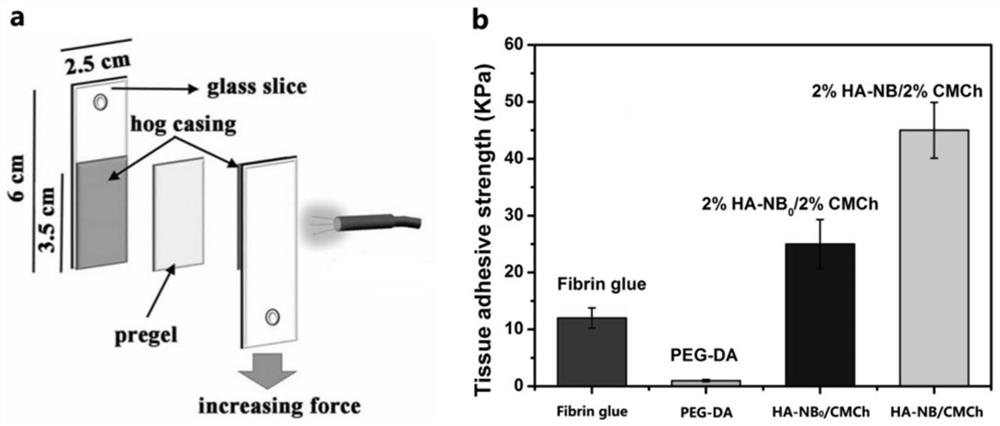
Preparation method, raw material, product and application of photocoupled crosslinked hydrogel material
A hydrophilic and water-soluble technology, applied in the field of biological materials, it can solve the problems of short photocuring wavelength, slow photocrosslinking speed (the initial gel formation time is about 30s, and limiting the clinical transformation of non-radical photocoupling crosslinking technology).
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0141] Embodiment one: the synthesis of component A-1
[0142]
[0143] (1) Synthesis of compound 1: synthesized according to the method disclosed in references Sarit S. Agasti.; Apiwat Chompoosor.; Vincent M. Rotello. J. Am. Chem. Soc. 2009, 131, 5728.
[0144] (2) Synthesis of compound 2: Dissolve compound 1 (1g, 2.9mmol) and ethylenediamine (1.1mL) in methanol (50mL), reflux for overnight reaction, rotary evaporation under reduced pressure, and dissolve the crude product in methanol , and reprecipitated from ethyl acetate. Compound 2 (0.92 g, yield 85%) was obtained after several times of dissolution-reprecipitation, filtration and vacuum drying. 1 H NMR (400MHz, CDCl 3 ):δ=7.91(s,1H),4.96(s,2H),4.13(t,J=6.1Hz,2H),3.99(s,3H),3.32(dd,J=11.6,5.7Hz,2H) ,2.82(t,J=5.9Hz,2H),2.44(t,J=7.2Hz,2H),2.26-2.17(m,2H).MS(ESI):[M+H]373.1373.
[0145] (3) Synthesis of component A-1: dissolve hyaluronic acid (2 g, 340 kDa) in 100 mL of 0.01 mol / L 2-(N-morpholine) ethanesulfonic acid...
Embodiment 2
[0146] Embodiment two: the synthesis of component A-2
[0147]
[0148] (1) Synthesis of compound 3: synthesized according to the method disclosed in references James F.Cameron.; JeanM.J.Frechet.J.Am.Chem.Soc.1991,113,4303.
[0149] (2) Synthesis of compound 4: Dissolve compound 3 (1g, 2.8mmol) and ethylenediamine (1.1mL) in methanol (50mL), reflux for overnight reaction, rotary evaporation under reduced pressure, and dissolve the crude product in methanol , and reprecipitated from ethyl acetate. After several times of dissolution-reprecipitation, filtration and vacuum drying gave compound 4 (0.86 g, yield 80%). 1 H NMR (400MHz, CDCl 3 ):δ=7.91(s,1H), 4.96(m,1H), 4.13(t,J=6.1Hz,2H), 3.99(s,3H),3.32(dd,J=11.6,5.7Hz,2H) ,2.82(t,J=5.9Hz,2H),2.44(t,J=7.2Hz,2H),2.26-2.17(m,2H),1.33(d,J=6.9Hz,3H).MS(ESI) :[M+H]387.1553.
[0150] (3) Synthesis of component A-2: dissolve hyaluronic acid (2 g, 340 kDa) in 100 mL of 0.01 mol / L 2-(N-morpholine) ethanesulfonic acid MES buffer solu...
Embodiment 4
[0151] Embodiment four: the synthesis of component A-4
[0152]
[0153] (1) Synthesis of compound 7: synthesized according to the method disclosed in references Isabelle Aujard.; Chouaha Benbrahim.; Ludovic Jullien. Chem. Eur. J. 2006, 12, 6865.
[0154] (2) Synthesis of compound 8: Dissolve compound 7 (1g, 2.7mmol) and ethylenediamine (1.1mL) in methanol (50mL), reflux for overnight reaction, rotary evaporation under reduced pressure, and dissolve the crude product in methanol , and reprecipitated from ethyl acetate. Compound 8 (0.95 g, yield 88%) was obtained after several times of dissolution-reprecipitation, filtration and vacuum drying. 1 H NMR (400MHz, CDCl 3 ):δ=7.91(s,1H),4.96(s,1H),4.13(t,J=6.1Hz,2H),3.99(s,3H),3.32(dd,J=11.6,5.7Hz,2H) ,2.82(t,J=5.9Hz,2H),2.44(t,J=7.2Hz,2H),2.26-2.17(m,2H).MS(ESI):[M+H]398.1326.
[0155] (3) Synthesis of component A-4: Dissolve hyaluronic acid (2 g, 340 kDa) in 100 mL of 0.01 mol / L 2-(N-morpholine) ethanesulfonic acid MES buff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com