Application of ginsenoside Rh2 in preparation of medicines for preventing and treating inflammatory bowel diseases
A technology of inflammatory bowel disease and ginsenoside, which is applied in the field of biomedicine, can solve the problems of adverse reactions of flora disorder, affecting the treatment effect of patients, and limiting the clinical efficacy of inflammatory bowel disease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
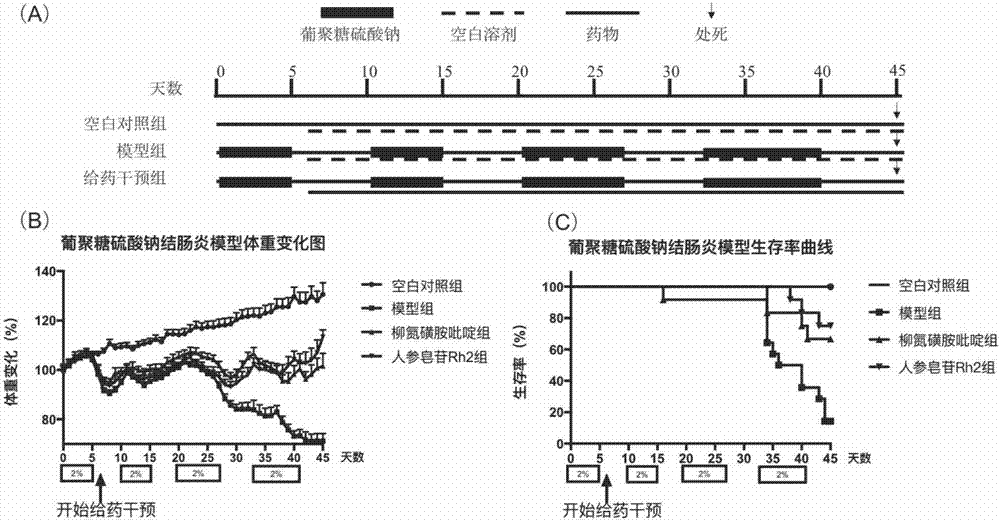
[0061] Dextran Sulfate Sodium Enteritis Mice Modeling: After C57 / B6 mice were raised in the animal room for 7 days, 2% dextran sulfate sodium solution was given in the drinking water of the mice for 5 days, and the mice were replaced every 2 days. The prepared dextran sodium sulfate solution, and then return to normal drinking water for 5 days, this 10 days is a cycle, lasting 2 cycles, and then give the dextran sodium sulfate solution with a mass concentration of 2% for 7 days, changing every 2 days Reconstituted dextran sodium sulfate solution, and then resume normal drinking water for 5 days, this 12 days is a cycle, lasting 2 cycles. For a total of 4 cycles of feeding dextran sodium sulfate solution, the mice were weighed daily. Then the mice were randomly divided into four groups: blank group, model group, ginsenoside Rh2 group, sulfasalazine SASP group. From the 6th day after modeling, the drug administration intervention started: Rh2 group was given 50 mg / kg ginsenosid...
Embodiment 2
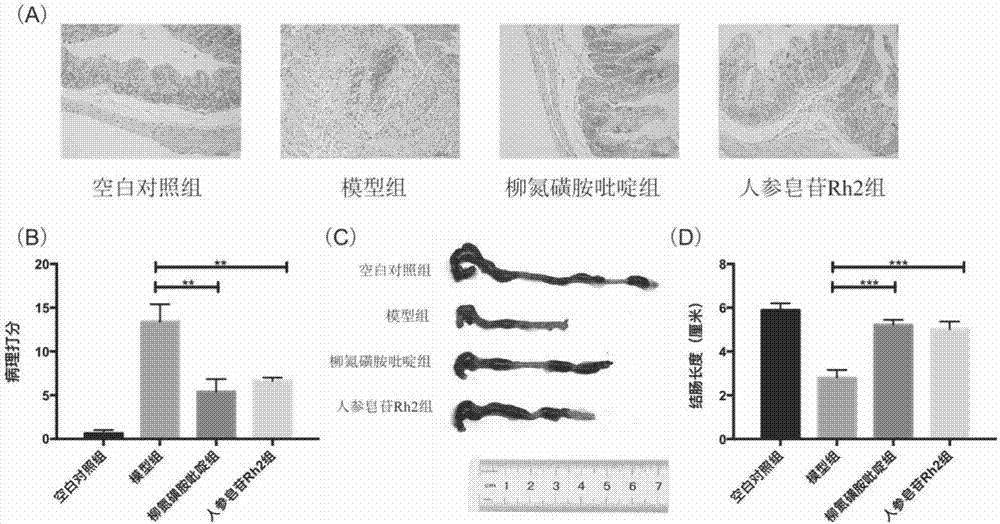
[0066] For details on the modeling method of dextran sodium sulfate enteritis mice, the method of feeding the mice and the method of killing the mice, see Example 1. Colon tissue was dissected in a clean bench, and the length of the colon was measured and photographed. The distal 0.5 cm of the colon was taken and washed with normal saline, fixed with 4% paraformaldehyde for 24 hours, then dehydrated, embedded in paraffin, sliced, and stained with HE. Dehydration, embedding in paraffin, slicing and HE staining were performed using conventional methods. Yes, see pathological section results figure 2 .
[0067] Histological scoring: Observe under a microscope and develop histological scoring criteria according to the literature:
[0068] ① Lesion range: none, 0 points; 1-25%, 1 point; 26-50%, 2 points; 51-75%, 3 points; 76-100%, 4 points;
[0069] ② Depth of lesion: none, 0 points; upper mucosa, 1 point; lamina propria, 2 points; mucosa and submucosa, 3 points; muscularis and...
Embodiment 3
[0077] Colon tissue inflammatory factor gene expression:
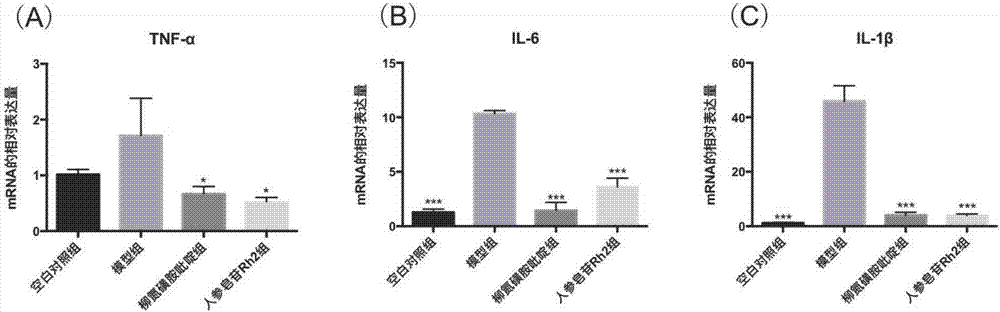
[0078] For details on the modeling method of dextran sodium sulfate mice, the method of feeding the mice and the method of killing the mice, see Example 1. The colon tissue was dissected, rinsed, 0.5 mg was weighed, an appropriate amount of Trizol was added, RNA was extracted according to the Takara total RNA extraction kit, and RNA was quantified. The mRNA was reverse transcribed into cDNA according to the instructions of the RT-PCR kit. PCR experiments were performed according to the instructions of the ThermalCycler DiceTM Real TimeSystem (TakaRa Code: TP800), and the results are shown in image 3 . The quantitative PCR reaction conditions were:
[0079] Stage1: Pre-denaturation: 95℃, 30sec;
[0080] Stage2: PCR reaction: 95℃, 10sec; 60℃, 30sec; 72℃, 30sec; 40cycles;
[0081] Stage3: Melting curve analysis: 95°C, 15sec; 70°C, 15sec.
[0082] according to image 3It can be concluded that (A) is the relative ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com