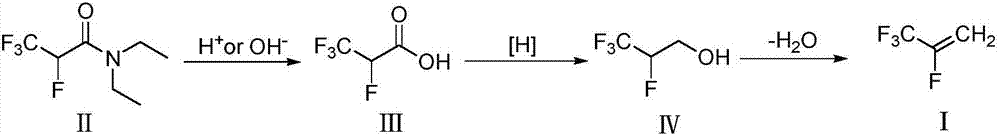Method for preparing 2,3,3,3-tetrafluoropropene
A technology of tetrafluoropropene and tetrafluoropropanol, which is applied in the field of preparation of 2,3,3,3-tetrafluoropropene, can solve the problems of waste of fluorine element and low utilization rate of fluorine element, and achieves low equipment requirements and conditions. Mild, easy-to-industrial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Hydrolysis reaction:
[0039] Add 603.5g (3.0mol) of N,N-diethyl-2,3,3,3-tetrafluoropropionamide and 440.6g (5.0mol) of 1,4-dioxane into a 3000mL three-necked glass reaction flask, mechanically Add dropwise 85% sulfuric acid solution 691.8g (H 2 SO 4 6.0mol), after the drop, the temperature rose to 100°C, and the temperature was maintained for 10 hours. Fluoropropionic acid, the gas chromatography analysis purity is 98.5%, the yield is 87.1%.
[0040] Reduction reaction:
[0041] Add NaBH to 1000mL three-necked glass reaction flask 3 CN 31.4g (0.5mol), tetrahydrofuran 200g (4.35mol), heated up to 40°C under magnetic stirring, added 73.0g (0.5mol) of 2,3,3,3-tetrafluoropropionic acid dropwise, and kept the reaction at 50°C after dropping 3 hours. After the reaction is complete, lower the temperature to 10°C, add 200g of deionized water dropwise, extract the reaction solution with ethyl acetate (100mL×3 times), combine the extracts, carry out atmospheric distillatio...
Embodiment 2
[0047] Hydrolysis reaction:
[0048] Add 603.5g (3.0mol) of N,N-diethyl-2,3,3,3-tetrafluoropropionamide and 440.6g (5.0mol) of 1,4-dioxane into a 3000mL three-necked glass reaction flask, mechanically Add 608g of concentrated hydrochloric acid (HCl6.0mol) dropwise under stirring, the internal temperature rises to 80°C after dropping, and keep it warm for 12 hours. 2,3,3,3-Tetrafluoropropionic acid has a gas chromatography purity of 97.7% and a yield of 73.0%.
[0049] Reduction reaction:
[0050] Add LiAlH to a 1000mL three-necked glass reaction flask 4 19g (0.5mol), 200g (4.35mol) of absolute ethanol, heated to 40°C under magnetic stirring, slowly added 73.0g (0.5mol) of 2,3,3,3-tetrafluoropropionic acid dropwise, and kept at 50°C after dropping React for 3 hours. After the reaction is complete, lower the temperature to 10°C, add 200g of deionized water dropwise, extract the reaction solution with ethyl acetate (100mL×3 times), combine the extracts, carry out atmospheric...
Embodiment 3
[0056] Hydrolysis reaction:
[0057] Add 603.5g (3.0mol) of N,N-diethyl-2,3,3,3-tetrafluoropropionamide and 54.0g (3.0mol) of deionized water into a 3000mL three-necked glass reaction flask, add 85 % sulfuric acid solution 691.8g (H 2 SO 4 6.0mol), the temperature rose to 100°C after dripping, and kept for 10 hours. After the reaction was completed, rectification at atmospheric pressure collected 119.5-120.5°C fractions to obtain 377.3g of colorless liquid, which was 2,3,3,3-tetra Fluoropropionic acid, the gas chromatography analysis purity is 99.0%, the yield is 85.3%.
[0058] Reduction reaction:
[0059] Add NaBH to 1000mL three-necked glass reaction flask 4 18.9g (0.5mol), 34.1g (0.25mol) of zinc chloride, 200g (4.35mol) of absolute ethanol, heated to 40°C under magnetic stirring, slowly added dropwise 73.0 g of 2,3,3,3-tetrafluoropropionic acid g (0.5 mol), after the dropwise reaction at 60°C for 3 hours. After the reaction is complete, lower the temperature to 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com