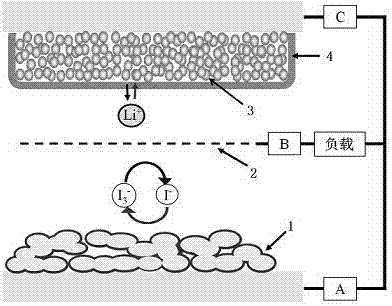
Photo-charging secondary battery taking phteropoly acid salt as negative electrode material
A technology of heteropoly acid salts and negative electrode materials, which is applied in battery electrodes, solar cells, circuits, etc., can solve the problems of unable to meet the large-scale storage of solar energy, and the performance cannot meet the requirements of battery applications, etc., and achieve low environmental hazards and high thermal stability. The effect of safety on sex and reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of nano ammonium phosphotungstate negative electrode material and electrode sheet:
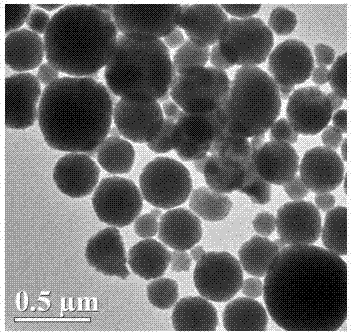
[0031] (1) Preparation of nano ammonium phosphotungstate: take 25ml, 0.19mol / L ammonium chloride solution, slowly add the ammonium chloride solution into 50ml, 0.09mol / L phosphotungstic acid solution, and the reaction temperature is 40℃ , Magnetic stirring speed 550r / min. The reaction time is 8h. Cool to room temperature after the reaction, wash and dry. Potassium phosphotungstate was prepared with nanometer microspheres. Its transmission electron microscope picture is shown in figure 2 .
[0032] (2) Mix the nano-ammonium phosphotungstate material prepared above, acetylene black and PVDF according to the weight ratio of 7:2:1, N-methylpyrrolidone is used as the dispersant, stir well to make the mixture uniform, and roll it into a sheet. Vacuum dry at 80°C for 12 hours for later use.
[0033] (3) Use the lithium iodide propylene carbonate solution prepared above as th...
Embodiment 2
[0035] Preparation of nano ammonium phosphomolybdate negative electrode material and electrode sheet:
[0036] Replace the phosphotungstic acid solution in step (1) in Example 1 with phosphomolybdic acid solution, and replace the reaction time of step (1) in Example 1 from 8h to 6h. In the step (2) of Example 1, nano-ammonium phosphotungstate was replaced with nano-ammonium phosphomolybdate, and the others were the same as in Example 1. The voltage-time curve when the light charging time is 20min and the discharge current density is 75mA / g is as follows Figure 5 shown. The discharge capacity-cycle life curve of the prepared nano-ammonium phosphotungstate negative electrode material is as follows: Figure 6 shown.
Embodiment 3
[0038] Preparation of nano-potassium silicotungstate material:
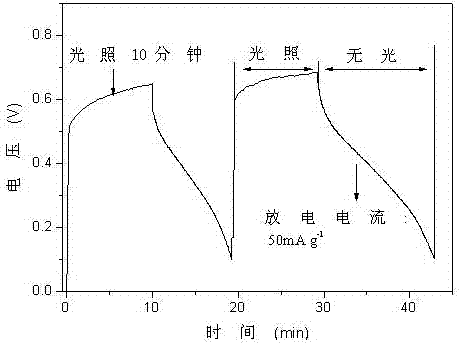
[0039] Replace the ammonium chloride solution in step (1) in Example 1 with potassium chloride solution, replace the phosphotungstic acid solution in step (1) in Example 1 with silicotungstic acid solution, and replace the solution in step (1) in Example 1 with In (1) the reaction temperature is replaced from 40°C to 60°C, and the reaction time in (1) in Example 1 is replaced from 8h to 4h. In the step (2) of Example 1, nano ammonium phosphotungstate was replaced by nanometer potassium silicotungstate, and the others were the same as in Example 1. The voltage-time curve when the light charging time is 30min and the discharge current density is 50mA / g is as follows Figure 7 shown. The discharge capacity-cycle life curve of the prepared nano-potassium silicotungstate negative electrode material is as follows: Figure 8 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com