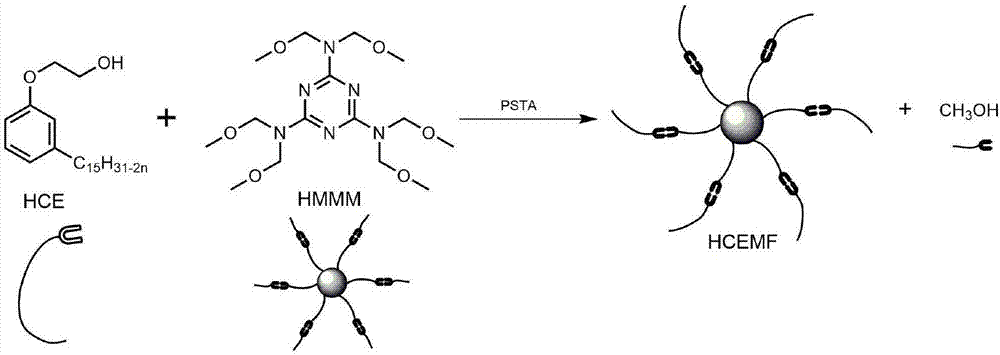
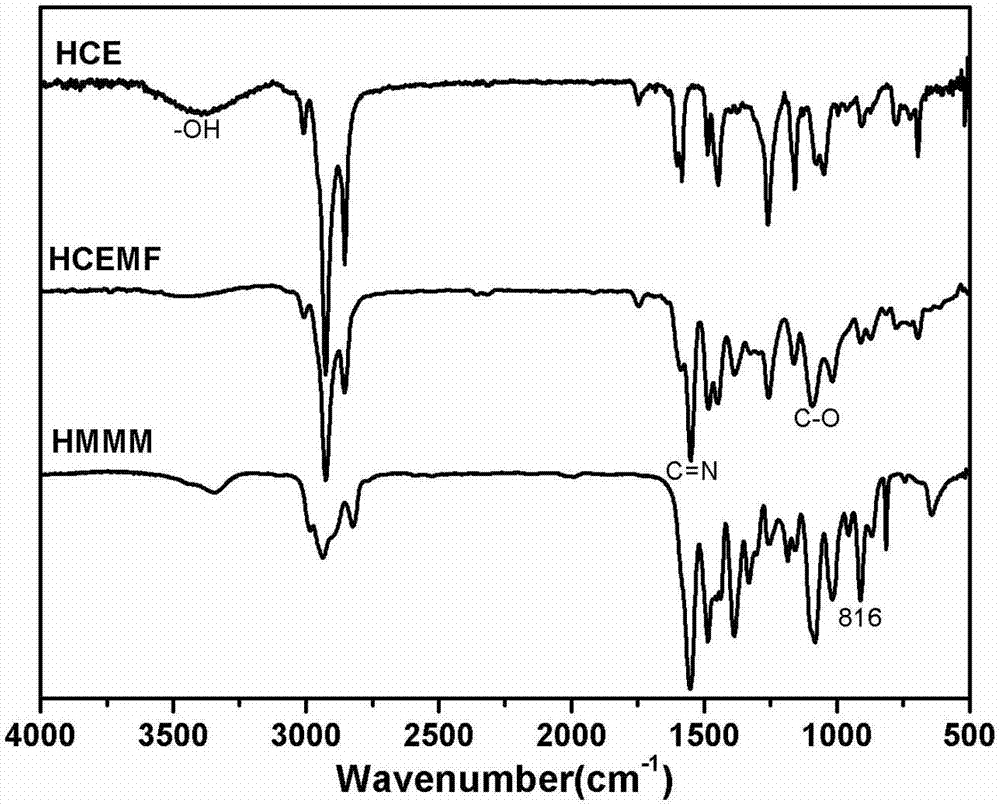
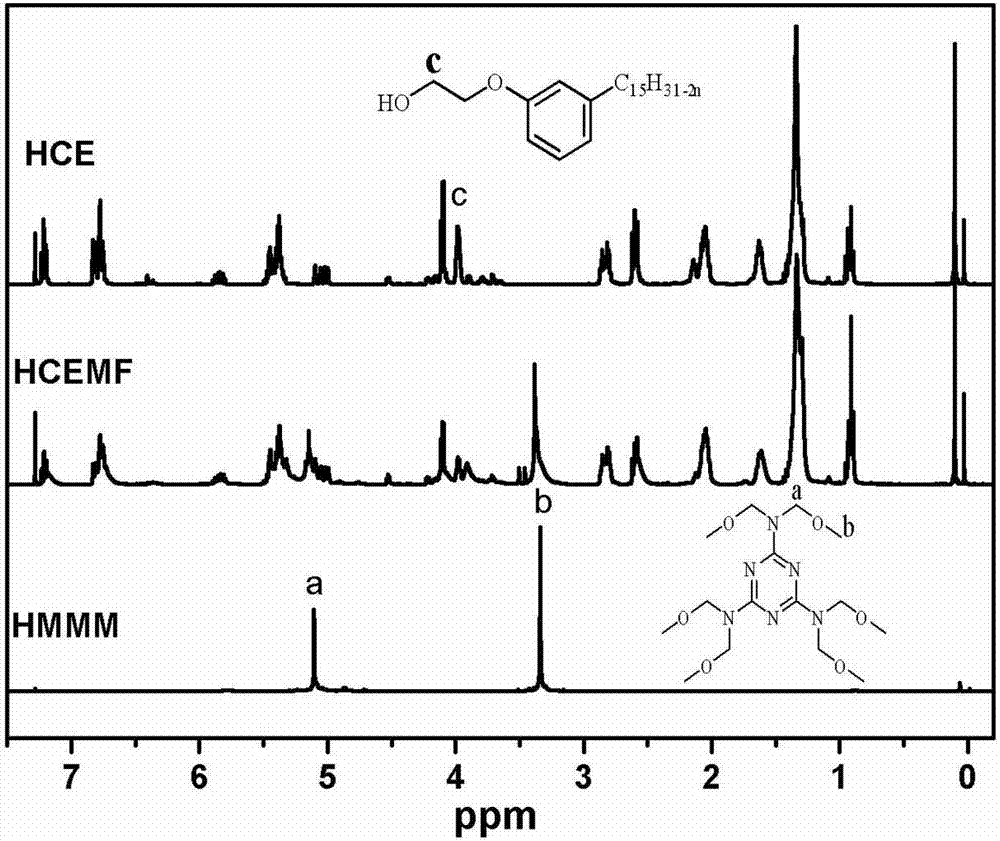
High-performance etherified biological base resin and preparing method thereof
A bio-based resin, amino resin technology, used in aldehyde/ketone condensation polymer adhesives, adhesive types, papermaking, etc., can solve the problem of easy self-crosslinking reaction, poor storage stability, and low etherification degree problems, to achieve the effect of improving market competitiveness and application value, high mechanical strength and hardness, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] (1) Add 23.426g high methyl etherified melamine formaldehyde resin (Jinan Sanqiang Chemical Co., Ltd., viscosity 7300mPa s), 124.034g hydroxyethyl cardanol ether, 0.468g p-toluenesulfonic acid, 12g n-octane Power stirring bar, condensing reflux tube, thermometer and 250mL four-necked flask equipped with n-octane water separator;
[0062] (2) Pass dry gas before heating up, slowly raise the temperature to 110°C and slightly reflux within 1 hour, and continue the reaction for 14 hours, so that n-octane and methanol generated in the reactant form an azeotrope to slowly remove from the reaction system Remove small alcohol molecules, react for 14 hours, then raise the temperature to 125°C and continue the reaction for 4 hours;
[0063] (3) Stop heating after the reaction is over and continue to stir and cool to room temperature, and remove residual n-heptane in the system by distillation under reduced pressure to obtain product A;
[0064] (4) Product A is washed repeatedly...
Embodiment 2
[0072] (1) Add 3.254g high-etherified melamine formaldehyde resin (Jinan Sanqiang Chemical Co., Ltd.), 17.227g hydroxyethyl cardanol ether, 0.069g p-toluenesulfonic acid, 2.146g isooctane to a power stirring bar, Condensation reflux tube, thermometer and 100mL four-neck flask equipped with n-heptane water separator;
[0073] (2) Pass dry gas before heating up, slowly raise the temperature to 105°C within 1.5 hours and reflux slightly, and continue the reaction for 13 hours, so that isooctane and butanol generated in the reactants form an azeotrope to slowly remove from the reaction system Removal of alcohol small molecules;
[0074] (3) Stop heating after the reaction is over and continue to stir and cool to room temperature, and remove residual isooctane in the system by distillation under reduced pressure to obtain product A;
[0075] (4) Product A was washed multiple times with acetonitrile to remove unreacted catalyst p-toluenesulfonic acid and raw materials, and after va...
Embodiment 3
[0080] (1) Add 23.614g of high methyl etherified urea formaldehyde resin, 137.816g of hydroxyethyl cardanol ether, 0.274g of p-toluenesulfonic acid, and 12g of n-octane into the In the 100mL four-neck flask of the alkanes water separator;
[0081] (2) Pass dry gas before heating up, slowly raise the temperature to 110°C and microreflux within 1.5 hours, and continue the reaction for 12 hours, so that n-octane and methanol generated in the reactants form an azeotrope to slowly remove from the reaction system Removal of alcohol small molecules;
[0082] (3) Stop heating after the reaction is over and continue stirring to cool to room temperature, and decompressed distillation to remove residual n-octane in the system to obtain product A;
[0083] (4) Product A was washed several times to remove unreacted catalyst and raw materials, distilled under reduced pressure, and dried in a vacuum oven for 24 hours to obtain the product modified urea-formaldehyde resin.
[0084] Highly e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com