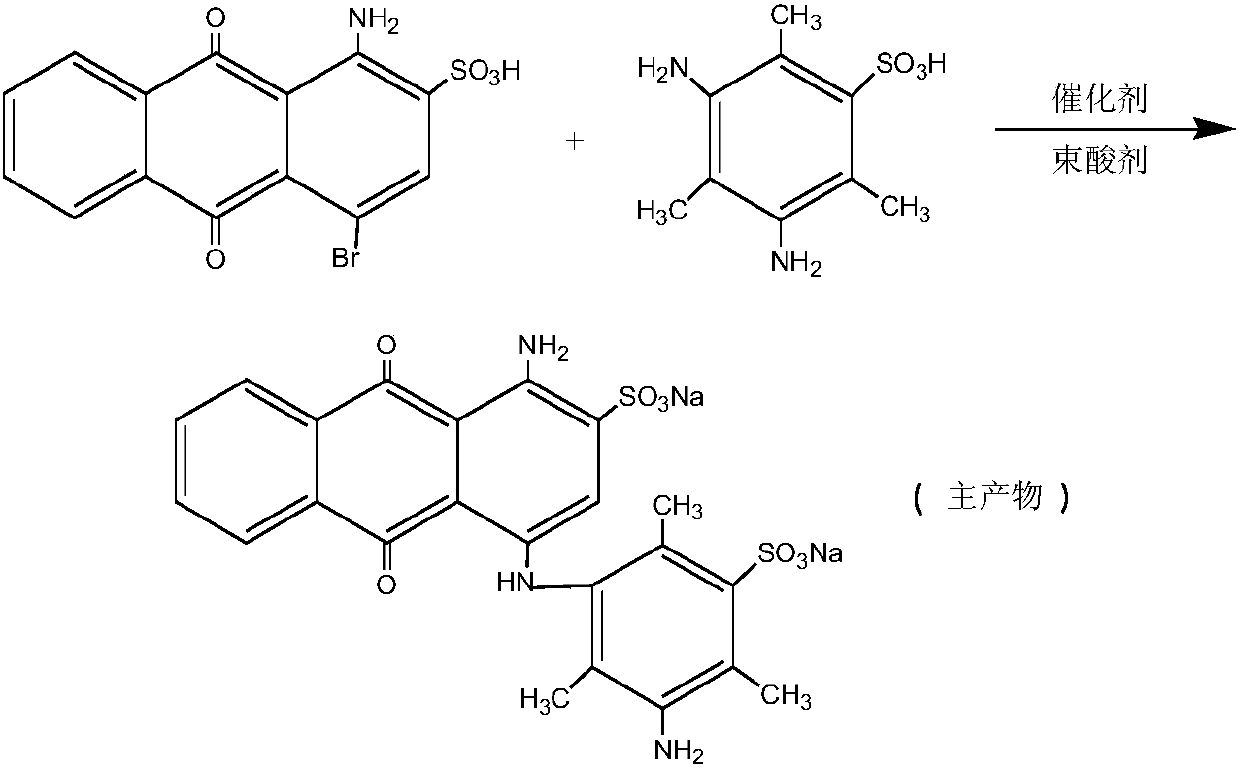
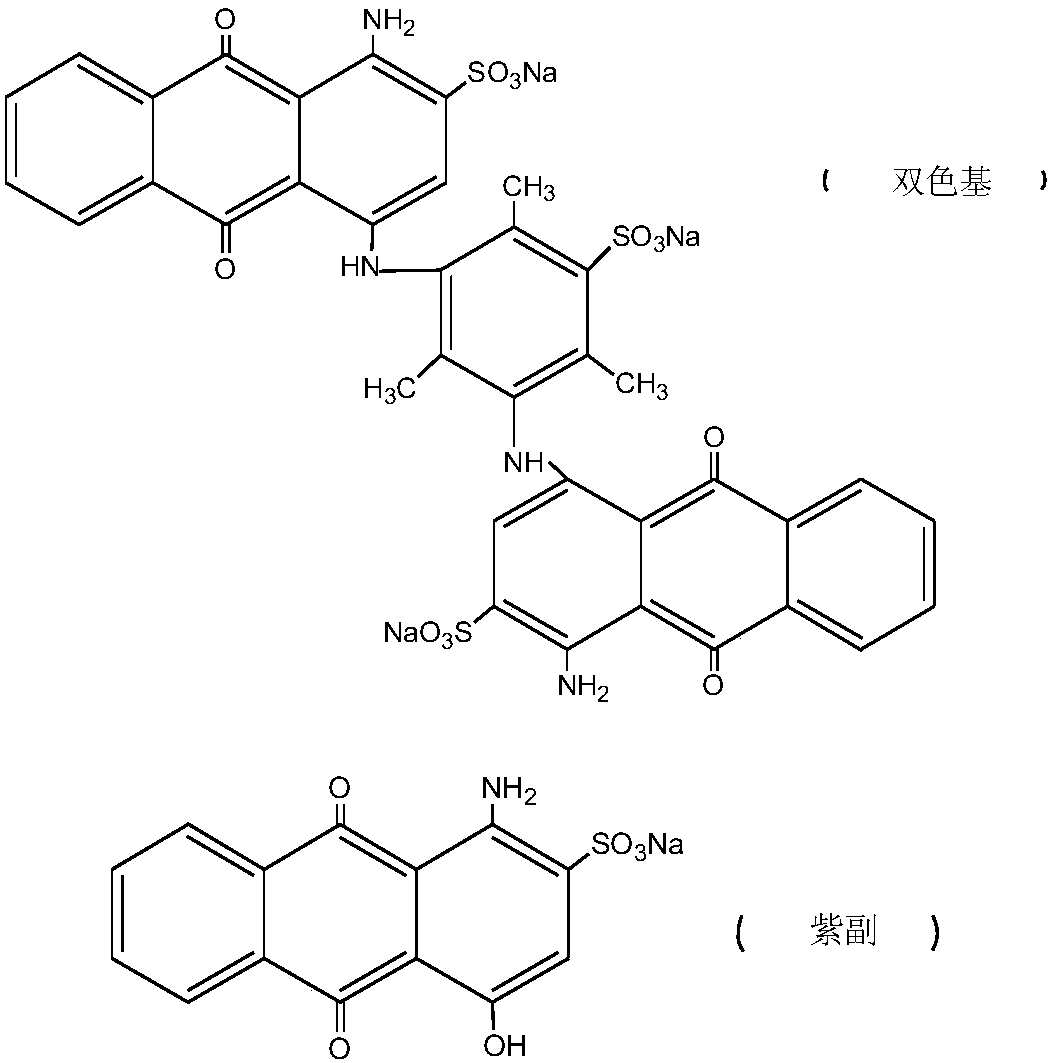
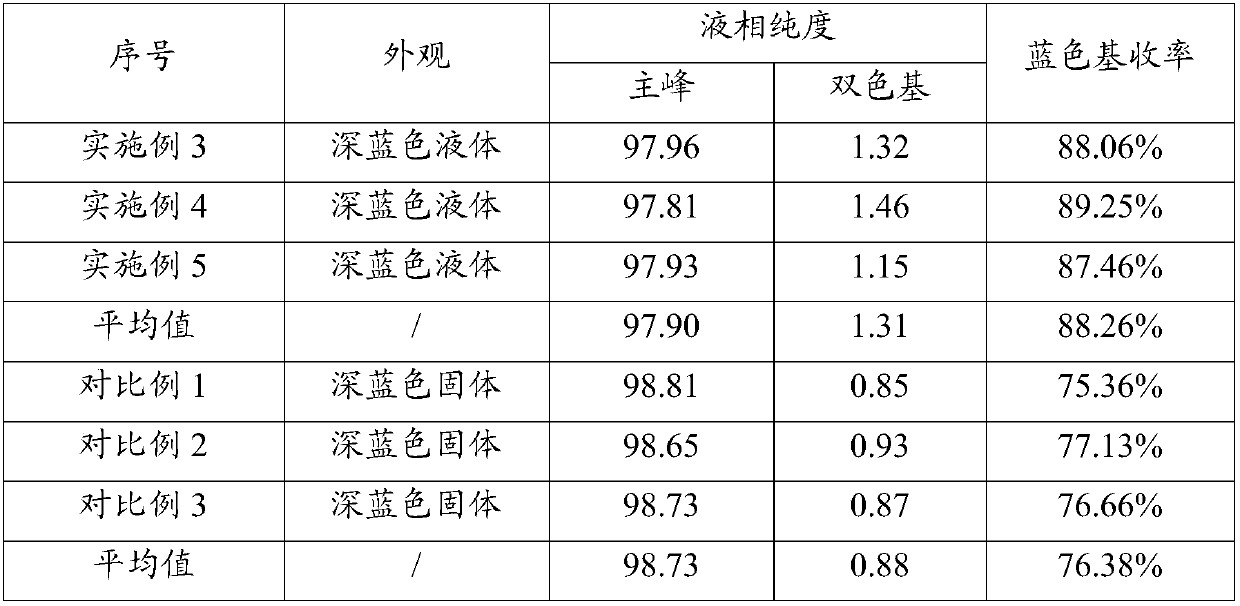
Preparation method of water-soluble blue printing dye
A water-soluble, blue technology, which is applied in the direction of anthracene dyes, organic dyes, chemical instruments and methods, etc., can solve the problems of reduced performance of dye application, influence of dye application, difficulty of bromidine, etc., and achieve improvement of dye application performance and increase Catalyst efficiency, the effect of reducing the amount of catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of solid-supported copper catalyst (copper / hollow ceramic ball)
[0034] The hollow ceramic ball was immersed in a hydrochloric acid solution, magnetically stirred at room temperature for 11 hours, washed with deionized water to pH 7 several times, filtered with suction, dried at 155°C, and placed in a desiccator for activation. Put the hollow ceramic ball into a muffle furnace at 300°C for thermal activation for 2.5 hours, and put it in a desiccator to cool for use. Mix a certain amount of copper powder with acrylic acid, stir at 4°C for 8 hours, and finally add a certain amount of activated hollow ceramic balls, raise the temperature to 70°C, continue to stir for 10 hours, and finally put it into a muffle furnace for 300°C calcination 2 Within hours, the copper / hollow ceramic ball catalyst is obtained.
Embodiment 2
[0035] Example 2: Preparation of solid-supported cuprous chloride catalyst (cuprous chloride / hollow ceramic balls)
[0036] The hollow ceramic ball was immersed in a hydrochloric acid solution, magnetically stirred at room temperature for 11 hours, washed with deionized water to pH 7 several times, filtered with suction, dried at 155°C, and placed in a desiccator for activation. Put the hollow ceramic ball into a muffle furnace at 300°C for thermal activation for 2.5 hours, and put it in a desiccator to cool for use. Mix a certain amount of cuprous chloride with anhydrous methanol, add a certain amount of activated hollow ceramic balls, raise the temperature to about 50°C, stir for 5.5 hours, distill under reduced pressure to remove excess methanol, and finally put the catalyst in a muffle furnace at 210°C Calcined for 5 hours, the cuprous chloride / hollow ceramic ball catalyst is obtained.
Embodiment 3
[0038] 1) Blue base intermediate liquid
[0039] Add 100mL of water to a 500mL four-necked flask, add 45.86 grams of M acid, heat up to 60°C, add 30% of the process mass fraction of liquid caustic soda, adjust pH 9.0, M acid is completely dissolved; 38.2 grams of bromamine acid, stir evenly, add the technological amount of baking soda, control the pH to 8.0, heat up to 75°C, add 0.5 grams of solid-supported copper catalyst, and then divide into 4 times and add 0.5 grams of solid-loaded chlorine every 30 minutes. Cuprous oxide catalyst 2.0g, keep for 13 hours, make up 100mL of water, add diatomaceous earth and stir for 20 minutes, filter, continue to use hydrochloric acid to adjust the pH of the filtrate to 1.7, filter and recover M acid for the next batch of blue base synthesis, filtrate Adjust the pH to neutral with soda ash powder and set aside.
[0040] 2) Secondary condensation
[0041] Weigh 18.45 grams of cyanuric chloride and 0.2 grams of dispersant MF in a 1000mL beaker for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com