Drug-release controlled dry suspension containing medical resin compound and preparation method thereof
A dry suspension and compound technology, applied in the field of pharmaceutical preparations, can solve the problems of single dosage form and increased workload of skeleton-type sustained-release preparations, and achieve the effects of fast drug release, low equipment requirements, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: preparation of drug-ion exchange resin complex
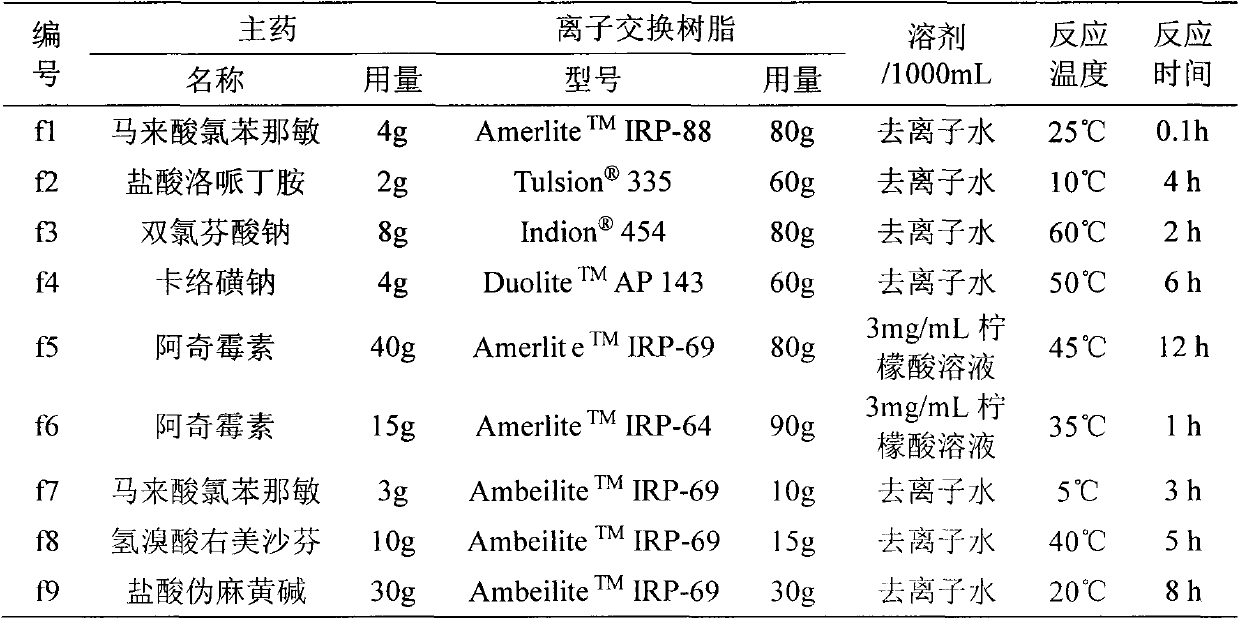
[0029] The preparation process of the drug-ion exchange resin complex is as follows: dissolve a certain amount of drug in 1000mL of the corresponding solvent, then add a certain amount of ion exchange resin, react at the corresponding temperature for a certain period of time, filter, and wash the product with deionized water , and dry to obtain the drug-ion exchange resin complex. See Table 1 for the dosage and conditions for the preparation of the corresponding compounds of each label.
[0030] Table 1 Conditions for the preparation of drug-ion exchange resin complexes
[0031]
Embodiment 2
[0032] Embodiment 2: Modification of drug-ion exchange resin complex
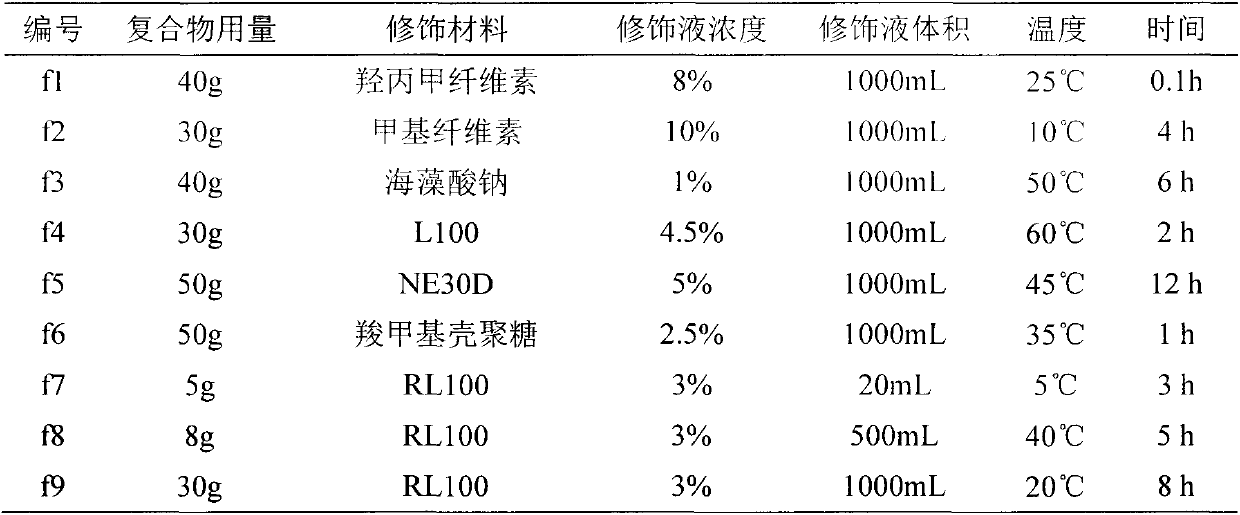
[0033] The modification process of the drug-ion exchange resin compound is as follows: the modification material is dissolved in water or swelled in water to obtain a modification material solution, and then the drug-ion exchange resin compound of each number in Example 1 is added to the modification material solution and stirred, filtered , washing the product, and drying to obtain the modified drug-ion exchange resin complex. The complex modification conditions corresponding to each label are shown in Table 2.
[0034] Table 2 Modification conditions of drug-ion exchange resin complexes
[0035]
Embodiment 3
[0036] Example 3: Drug loading before and after modification of the drug-ion exchange resin complex
[0037] The drug-loading capacity of each drug-ion exchange resin complex before and after modification was measured by dissociation method, and the measured results are shown in Table 3 below.
[0038] Table 3 Drug loading of drug-ion exchange resin complexes before and after modification
[0039]
[0040]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com