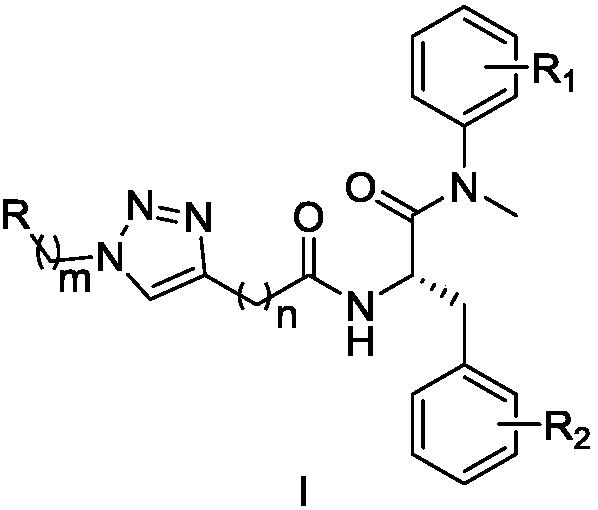
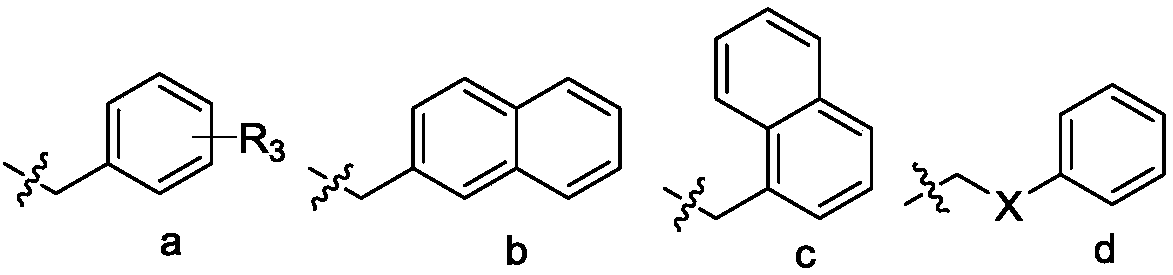
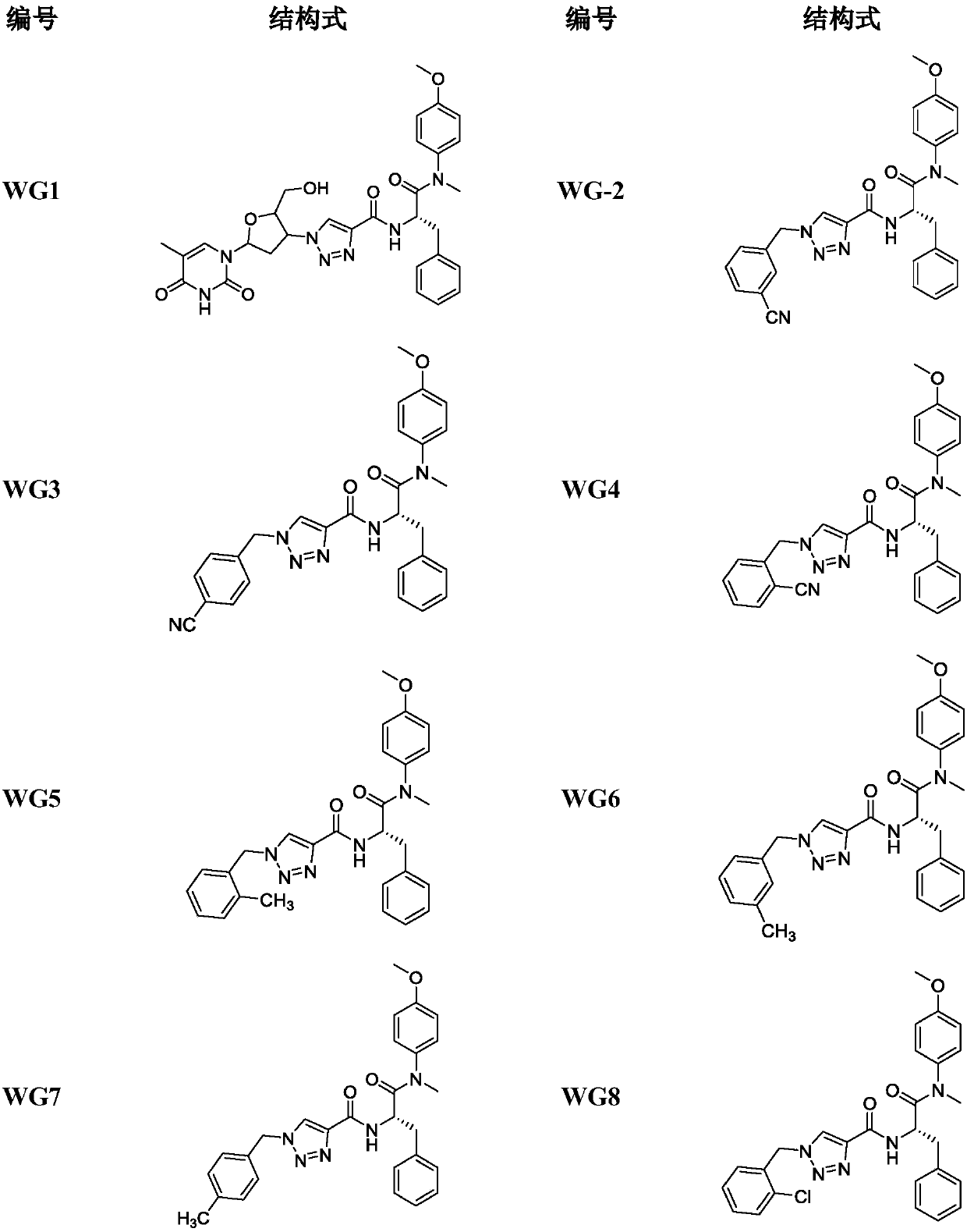
Triazole ring-containing phenylalanine derivative as well as preparation method and application thereof
A technology of phenylalanine and derivatives is applied in the fields of organic compound synthesis and medical application, and can solve the problems of low curative effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Key intermediate (S)-N-(1-(4-methoxyphenyl)(methyl)amino)-1-carbonyl-3-phenyl-2-yl)propynamide (4 ) preparation
[0056] The starting material Boc-L-phenylalanine (1) (8.75mmol, 2.3g) was added to 15mL of anhydrous dichloromethane solution, and then 1H-benzotriazol-1-yl was added to this solution Oxytripyrrolidinyl hexafluorophosphate (10.9 mmol, 5.7 g). Stir under ice bath conditions for 0.5h, then add N,N-diisopropylethylamine (21.87mmol, 3.61mL) and N-methyl-4-aminoanisole (7.29mmol, 1.0g), remove the ice bath , stirred at room temperature for 12h. After the reaction was completed, the solvent was evaporated under reduced pressure, then saturated sodium bicarbonate solution was added to the residue in the bottle, extracted with ethyl acetate; the organic layer was separated, 1N HCl solution was added, extracted with ethyl acetate; the organic layers were combined, saturated with Wash with brine, dry the organic phase with anhydrous sodium sulfate; filte...
Embodiment 2
[0059] Embodiment 2. the synthesis of target product WG1-WG20
[0060] The key intermediate 4 (0.297mmol, 1eq) and the prepared azide (0.357mmol, 1.2eq) were added into a mixed solvent of tetrahydrofuran and water (v / v=1:1, 10mL). Sodium ascorbate (0.0891 mmol, 17.65 mg) and copper sulfate pentahydrate (0.0297 mmol, 7.43 mg) were then added to this solution. The mixed solution was heated to 30-65°C and stirred vigorously for 4-12h. Subsequently, an appropriate amount of water was added to the reaction solution, extracted with ethyl acetate; the organic layer was separated and dried over anhydrous sodium sulfate; filtered and evaporated to dryness under reduced pressure; silica gel column chromatography; The target compounds WG1-WG20 were obtained by recrystallization from methane / n-hexane.
[0061]
[0062] The azide used was zidovudine. The product is a white solid, yield: 50%, melting point 143-145°C.
[0063] 1 H NMR (400MHz, DMSO-d 6 )δ11.37(s,1H),8.73(s,1H),8.35(...
Embodiment 3
[0120] Embodiment 3. In vitro anti-HIV-1 activity test (MT-4 cell) experiment of target compound
[0121] Principle: Luciferase reporter gene assay (HIV-1NL4-3 with nef gene deletion)
[0122] testing method:
[0123] Multicycle Virus Replication Assay in MT-4 Cells
[0124] Inoculated with MT-4 cells (1x 10 5cells / mL) on a 96-well cell culture plate, each well was added with different concentrations of target compounds (WG1-WG20 and PF-74), and then HIV-1NL4-3Nanoluc-sec virus strain (50TCID 50 / well) to infect MT-4 cells. The HIV-1NL4-3Nanoluc-sec used is a responder virus, which is inserted into the secNluc sequence in pNL1.3[secNluc] through the Not I and Xho I restriction endonuclease sites to replace the nuclear spanning DNA in the pNL4-3 plasmid. Nef sequence at nucleotides 8796-8892. Wherein, the Not I site is introduced into the pNL4-3 plasmid by the site-directed mutagenesis technique, and the Xho I site is a unique site existing in the pNL4-3 plasmid. After HI...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com