Preparation method of canrenone
A technology of canrenone and compounds, which is applied in the field of drug synthesis, can solve the problems of difficult complete reaction of products, low yield and purity, and achieve the effects of reduced side reactions, high yield, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
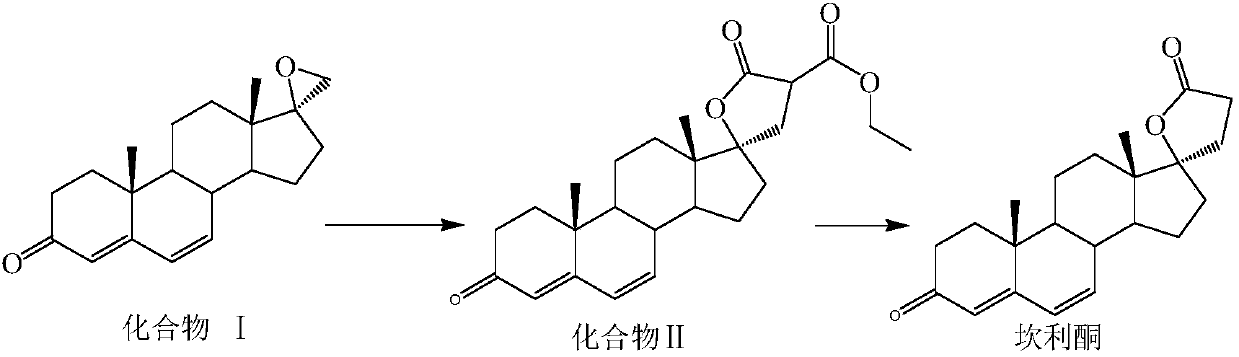
[0024] A kind of preparation method of canrenone, concrete experimental operation is as follows:
[0025] Under the protection of inert gas, add organic solvent, sodium ethoxide, diethyl malonate, and catalyst I to the reactor in sequence, stir for 0.3-1h, add compound I, keep warm for 4-7h, TLC shows that after the reaction is complete , lower the temperature to -5~5°C, add acid to adjust the pH to 7.0~7.2, concentrate most of the solvent under reduced pressure, add toluene to concentrate to dryness, then add toluene as the reaction solvent, transfer the reaction solution to the autoclave, add water, after sealing, replace the air with nitrogen, raise the temperature, after the reaction is complete, concentrate to dry toluene, add water to stir, suction filter, and dry to obtain canrenone.
[0026] Wherein: the volume mass ratio of diethyl malonate and compound I is diethyl malonate:compound I=(0.6~1.2)V:1W;
[0027] The volume mass ratio of sodium ethylate to compound I is ...
Embodiment 1
[0038] A preparation method of canrenone, the technical process is as follows:
[0039] The specific experimental steps are as follows:
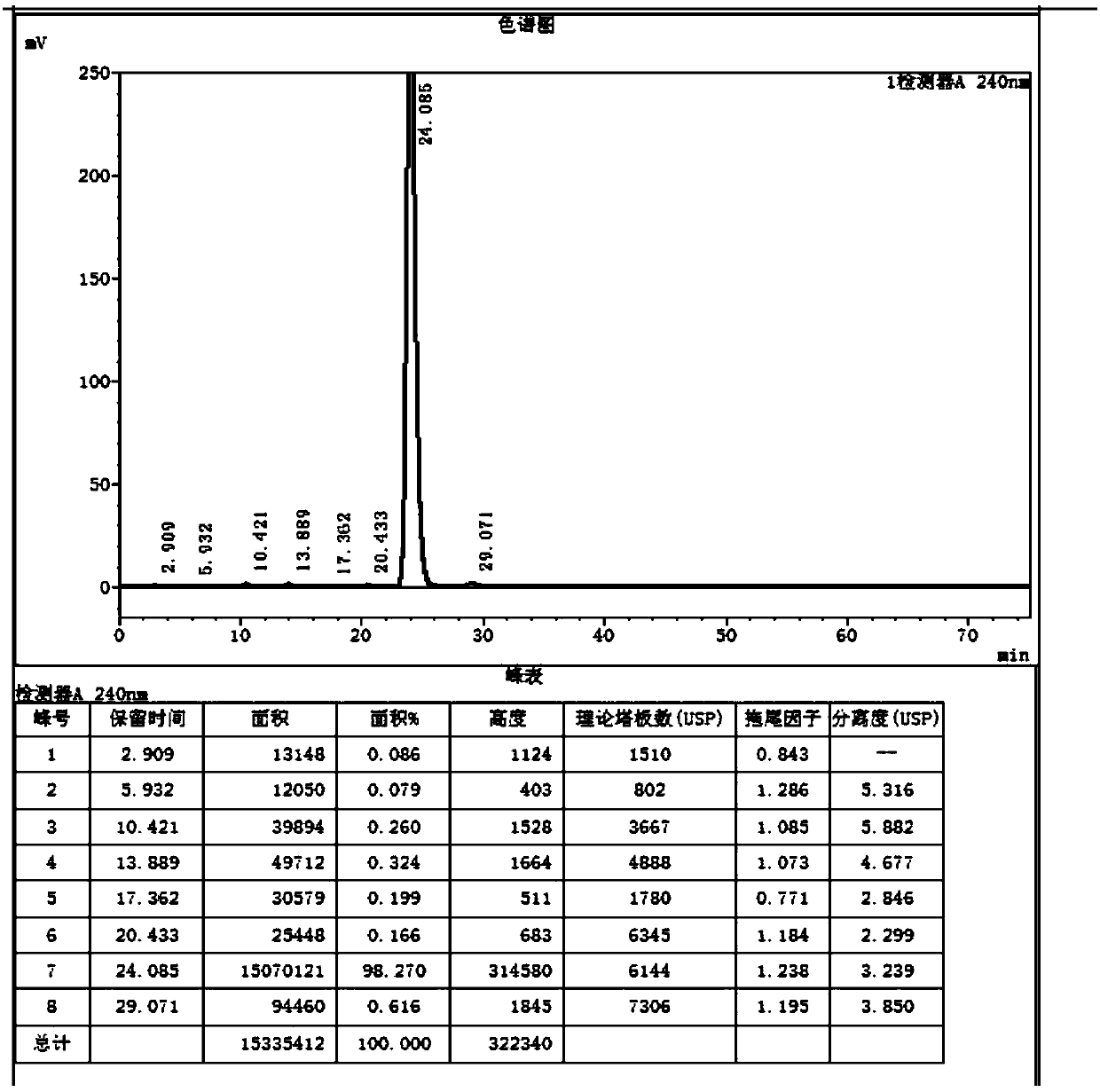
[0040] Under the protection of nitrogen, add 150ml of ethanol and 15g of sodium ethoxide to the three-necked flask, raise the temperature to 78°C, add 18ml of diethyl malonate, 0.15g of potassium dihydrogen phosphate, stir for 0.3h, add 30g of compound Ⅰ, keep the temperature for 4h, TLC (PE:EA=4:1) showed that the reaction was complete, lowered the temperature to -5°C, added an appropriate amount of glacial acetic acid to adjust the pH to 7.0, concentrated most of the solvent, added toluene to recover the remaining solvent, added 150ml of toluene, and transferred to In the autoclave, add 15ml of water, seal it and replace it with nitrogen three times, raise the temperature to 100°C, react for 24 hours, and the pressure is 2.5kg. After TLC (PE: EA = 4:1) shows that the reaction is complete, cool down, concentrate to viscous, add water to con...
Embodiment 2
[0042] A preparation method of canrenone, the technical process is as follows:
[0043] The specific experimental steps are as follows:
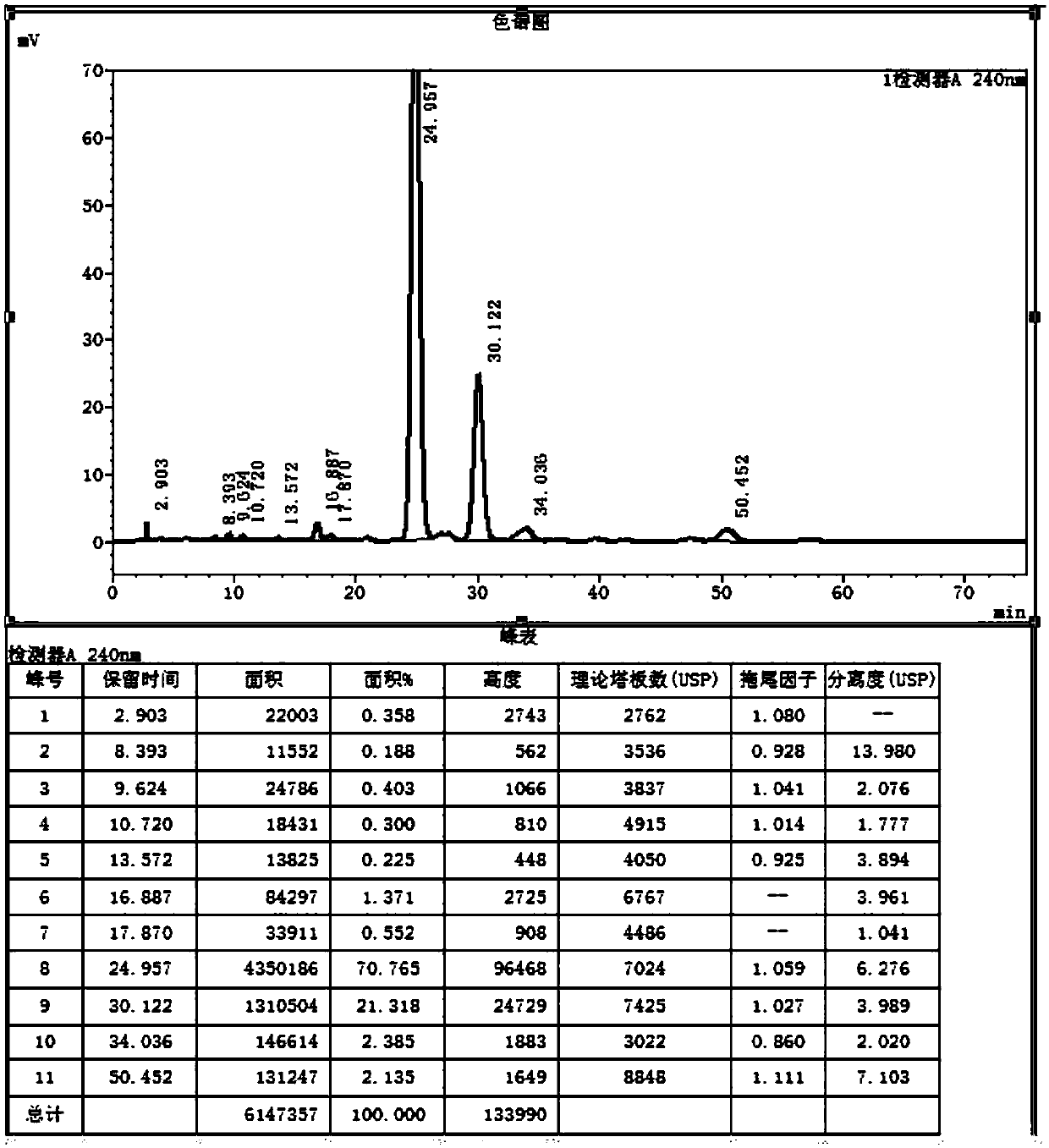
[0044] Under the protection of helium, add 150ml of methanol, 150ml of ethanol, 15g of sodium ethoxide into the three-necked flask, heat up to 78°C, add 60ml of diethyl malonate, 0.25g of potassium dihydrogen phosphate, stir for 0.5h, add 50g of compound Ⅰ, Insulate the reaction for 5 hours, TLC (PE:EA=4:1) shows that the reaction is complete, lower the temperature to 0°C, add an appropriate amount of glacial acetic acid to adjust the pH to 7.1, concentrate most of the solvent, add toluene to recover the remaining solvent, add 300ml of toluene , transfer to an autoclave, add 35ml of water, replace with helium three times after sealing, heat up to 120°C, pressure 3kg, react for 20h, TLC (PE:EA=4:1) shows that the reaction is complete, cool down, concentrate to viscous, Add water and continue to dry toluene, add appropriate amount of water an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com