Application of cholest-4-ene-3,6-dione in preparing drug for treating or preventing neuron injury
A technique for neuron damage and cholesteric, applied in drug combinations, nervous system diseases, neuromuscular system diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
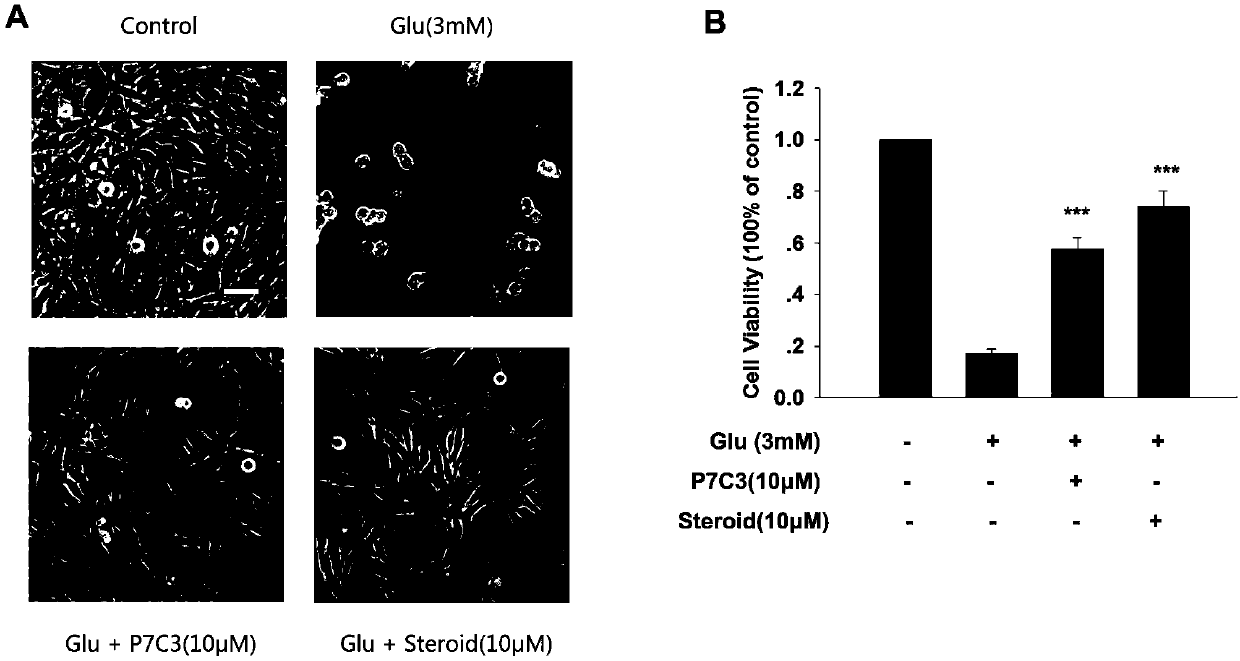
[0024] Example 1. Glutamate-induced HT-22 oxidative stress injury model
[0025] Take the HT-22 cells that have been passaged three times in a medium dish after resuscitating with liquid nitrogen. Before use, observe that the cells are in good condition, the cell bodies are round and full, the synapses are complete, and there are almost no dead cells. Digest with 0.5ml of 0.25% trypsin for half a minute , blown into a single-cell suspension, collected in a 15ml centrifuge tube, added complete medium to 3ml, centrifuged at 1000rpm for 3min, removed the supernatant, and evenly dispersed the precipitate in 4ml complete DMEM medium (DMEM+10%FBS+1%P / S), take 0.8ml of cell suspension for passage, then take an appropriate amount of suspension and add it to complete DMEM medium, dilute to 4*10 4 cells / ml, planted in a 48-well plate (200 μl / well), and used for experiments after 24 hours.
[0026] First, cholest-4-ene-3,6-dione was dissolved in DMSO to make a final concentration of 10...
Embodiment 2
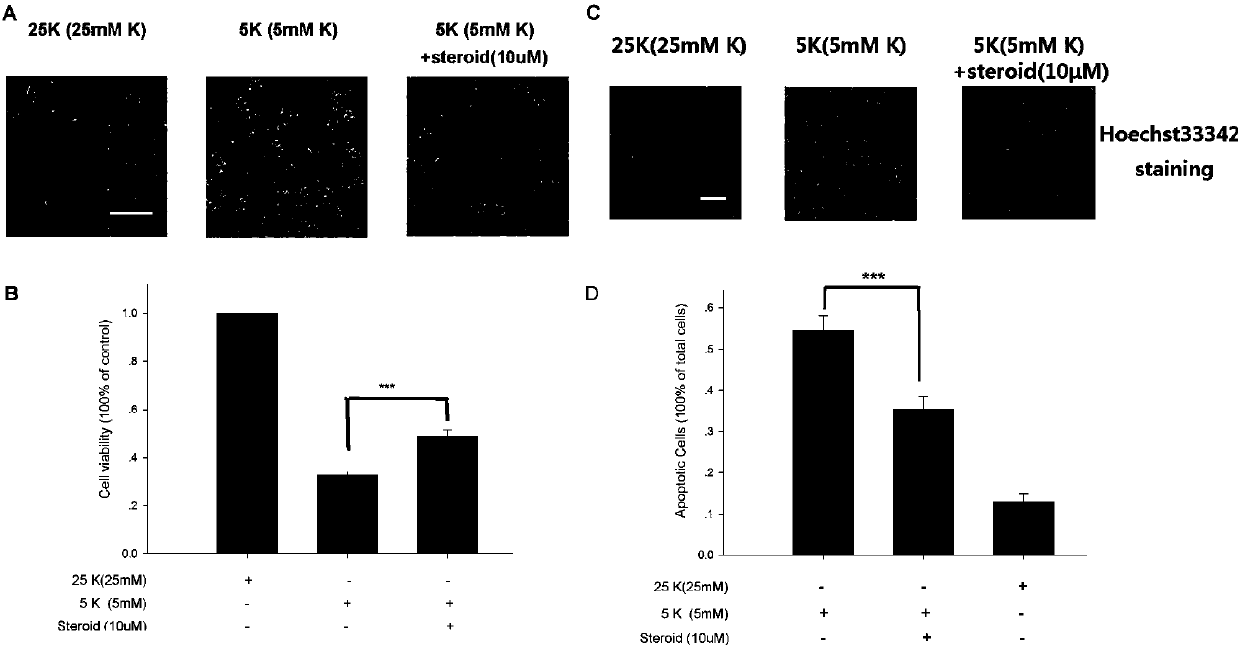
[0032] Example 2. Apoptotic injury model of cerebellar granule neurons induced by potassium deprivation
[0033] Take out 7-day-old rats, cut off their heads with major surgery, and use small ophthalmological scissors to cut from the vertebral foramen to the upper edges of the left and right ears, then use tweezers to open the upper layer of the brain to expose the brain tissue, take out the cerebellum tissue and put it in a container. Place DMEM without phenol red (for dissection fluid) in a medium dish on an ice pack.
[0034] After removing the meninges and blood vessels on the cerebellum with ophthalmic forceps, use tissue scissors to mince the cerebellar tissue. Transfer the tissue into trypsin with a concentration of 0.25%, digest at 37°C for 15 minutes, add complete BME medium to 15ml, then add 100μl of DNase I (8mg / ml), blow off the tissue mass with a dropper, and then Centrifuge at 1000rpm for three seconds, transfer the supernatant to another centrifuge tube, centri...
Embodiment 3
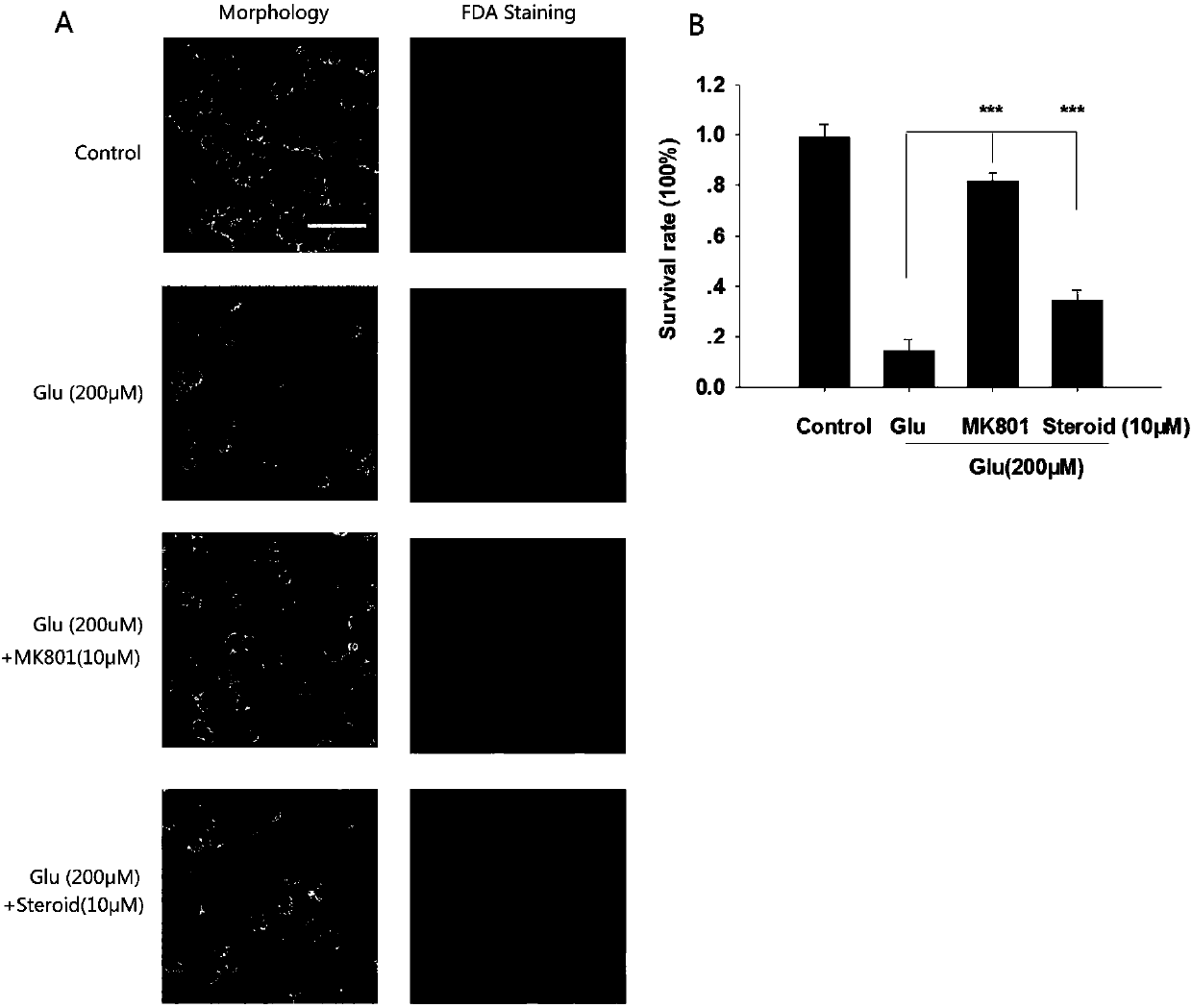
[0043] Example 3. Excitotoxicity model of cerebellar granule neurons induced by glutamate
[0044] Take the cerebellar granule neurons that have been cultured for 7 days, observe under the microscope, the cells are round, the synapses are obvious, and the distribution is even, that is, it can be used for experiments. Do the following group processing:
[0045] a) Control group: pre-applied without Mg 2+ Locke,s Buffer
[0046] b) Glutamate group: no Mg pre-applied 2+ Locke's Buffer+200μM Glu
[0047] c) MK801 group: pre-coated with 10 μM MK801 without Mg 2+ Locke's Buffer+200μM Glu
[0048] d) Cholester-4-ene-3,6-dione group: pre-coated with 10 μM steroid without Mg 2+ Locke's Buffer+200μM Glu
[0049] Take the cerebellar granule neurons cultured for 7 days, collect the BME-conditioned medium of each well, and use Mg-free 2+ Locke, sBuffer solution washed cells three times, and then Mg-free2+ The drug solution prepared in Locke's Buffer solution was pre-coated at 37°C ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com