Synthesis method of 1, 1, 1-trichloroethane
A technology of trifluoroethane and difluoroethane, which is applied in the field of synthesizing 1,1,1-trifluoroethane, which can solve the problems of failure to meet the requirements of anti-corrosion or low-corrosion use, reliability and safety hazards, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
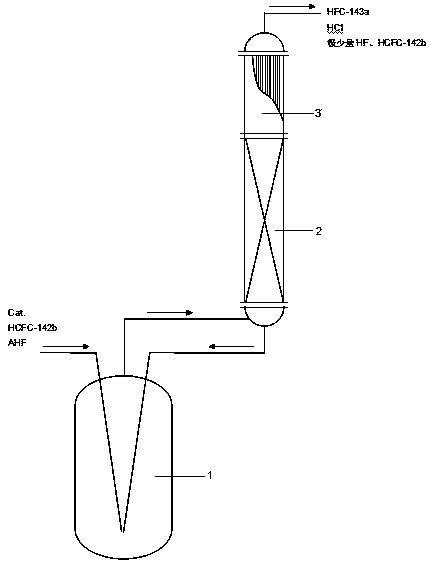
[0027] Sncl 4 As a catalyst to synthesize 1,1,1-trifluoroethane. The implementation process is as follows: add the Monel and Q345R steel of the pre-cleaned and dried scraper test sample to the bottom of the fluorination reactor 1, and after the fluorination reactor 1 is sealed, add 0.55kg of hydrogen fluoride and 2kg of Sncl 4Sequentially add to 15 liters of fluorination reactor 1 made of RQ345R steel, heat fluorination reactor 1 with water vapor, and make its internal temperature rise to 85 ° C, at this time, a certain amount of hydrogen chloride gas is produced by the catalyst activation reaction, from It is released from the discharge port, and then emptied after washing with water and alkali. The hydrogen chloride gas in the fluorination reactor 1 was exhausted, and the temperature was lowered to 50° C. with cooling water to obtain an active catalyst. Control the internal temperature of fluorination reactor 1 at 50°C, and add HCFC-142b at 1.6kg / hr and hydrogen fluoride a...
Embodiment 2
[0029] The present embodiment adopts the same operating method as in Example 1, and the catalyst and reaction conditions during the operation are listed below.
[0030] Add 2kg fluorosulfonic acid FS0 3 H as a catalyst. The results of this example are: the content of HFC-143a in the reaction product is 53.2%, the unreacted HCFC-142b is 46.8%, no high boiler tar and other by-products are detected, the annual corrosion rate of Monel alloy is 0.01mm, and the Q345R steel The annual corrosion rate is 0.1mm.
Embodiment 3
[0032] The present embodiment adopts the same operating method as in Example 1, and the addition and product conditions during the operation are listed below.
[0033] Add 1.5kgSncl 4 And 0.5kg fluorosulfonic acid as catalyst. The results of this example are: the content of HFC-143a in the reaction product is 99.8%, the unreacted HCFC-142b is 0.2%, no high boiler tar and other by-products are detected, the annual corrosion rate of Monel alloy is 0.01mm, and 16MnR is ordinary The annual corrosion rate of carbon steel is 0.15mm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com