Application of oxazolidinone-nitroimidazole coupling molecule
A technology of nitroimidazoles and oxazolidinones, which is applied in the field of medicine, can solve problems such as drug side effects, and achieve the effect of treating anaerobic infectious diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
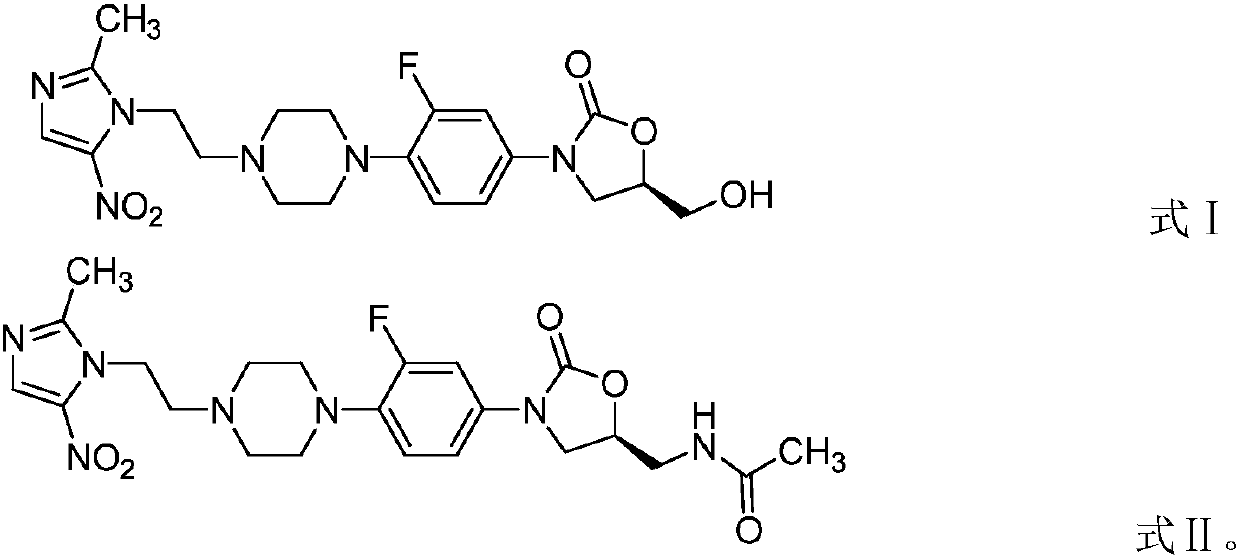
[0023] This example provides an application of an oxazolidinone-nitroimidazole coupling molecule in antibacterial applications. The oxazolidinone-nitroimidazole coupling molecule is a compound represented by formula I and / or formula II:
[0024]
[0025] In this example, the compounds represented by formula I and formula II were tested for in vitro antibacterial activity of standard strains to illustrate their general antibacterial ability. For the convenience of expression, the compound shown in formula I is called compound 1 in the examples (the sample in this example is selected from batch number DJ-013-014, produced by Dannuo Pharmaceutical), and the compound shown in formula II is called compound 2 (this example Examples Sample batch number DJ-013-032, produced by Dannuo Pharmaceuticals).
[0026] In this embodiment, the in vitro antibacterial activity test on 8 standard strains was firstly used, and the 8 strains were all from the American Type Culture Collection (ATC...
Embodiment 2
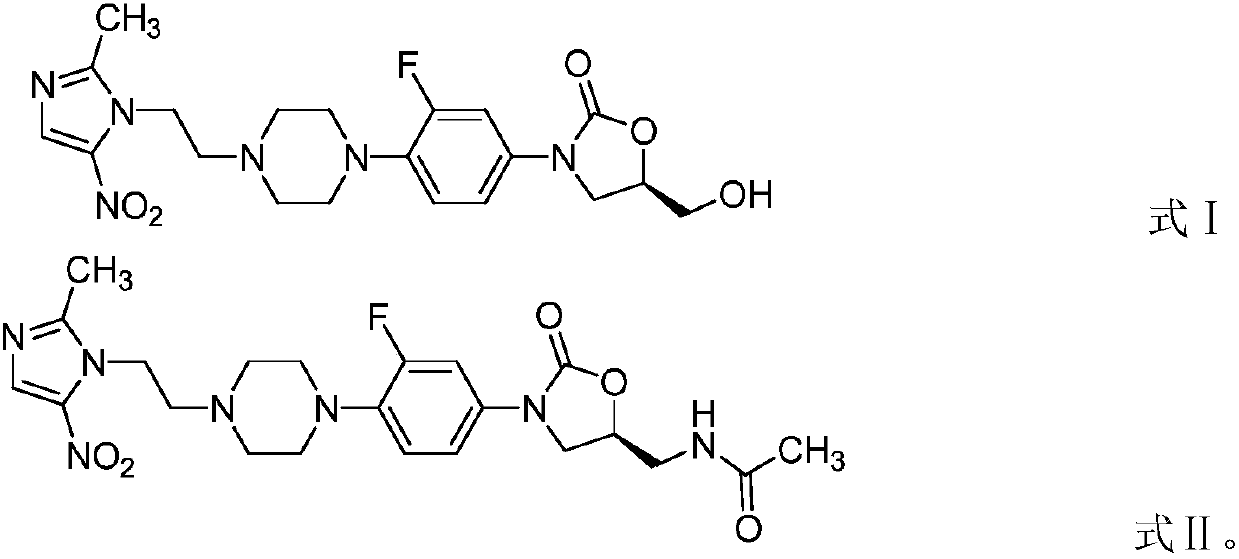
[0039] This example provides an application of an oxazolidinone-nitroimidazole coupling molecule in anti-anaerobic bacteria. The oxazolidinone-nitroimidazole coupling molecule is a compound represented by formula II:
[0040]
[0041] In this example, the compound represented by formula II is called compound 2 for convenience of description (the sample in this example is selected from batch number DJ-013-032, produced by Dannuo Pharmaceutical). This example is to test the in vitro antibacterial activity of compound 2 against various ribonucleic acid types of Clostridium difficile, in order to illustrate the inhibitory effect of compound 2 on Clostridium difficile.
[0042] In this embodiment, 28 strains of Clostridium difficile were selected for testing, as shown in Table 3. Twenty-eight strains of Clostridium difficile came from the American Type Culture Collection (ATCC) and the British National Type Culture Collection (NCTC). All strains were inoculated on Brucella agar...
Embodiment 3
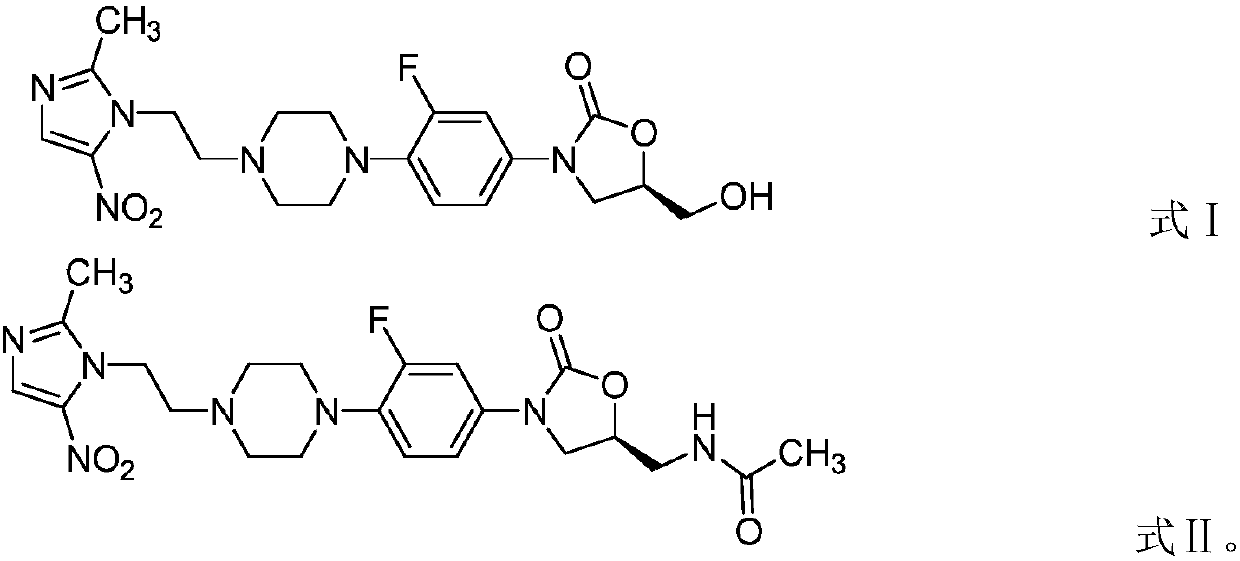
[0053] This example provides an application of an oxazolidinone-nitroimidazole coupling molecule in anti-anaerobic bacteria. The oxazolidinone-nitroimidazole coupling molecule is a compound represented by formula II:
[0054]
[0055] In this example, the compound represented by formula II is called compound 2 for convenience of description (the sample in this example is selected from batch number DJ-013-032, produced by Dannuo Pharmaceuticals, dissolved in DMSO). In this example, the antibacterial activity of Compound 2 against 29 different bacterial species including 20 anaerobic bacteria was tested.
[0056] The method of this embodiment is as follows:
[0057] Material:
[0058] Strains: 29 different bacterial species including: Gram-positive Enterococcus faecium, Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae; Gram-negative Escherichia coli, Haemophilus influenzae, Neisseria gonorrhoeae and Mycobacterium smegmatis; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mic value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com