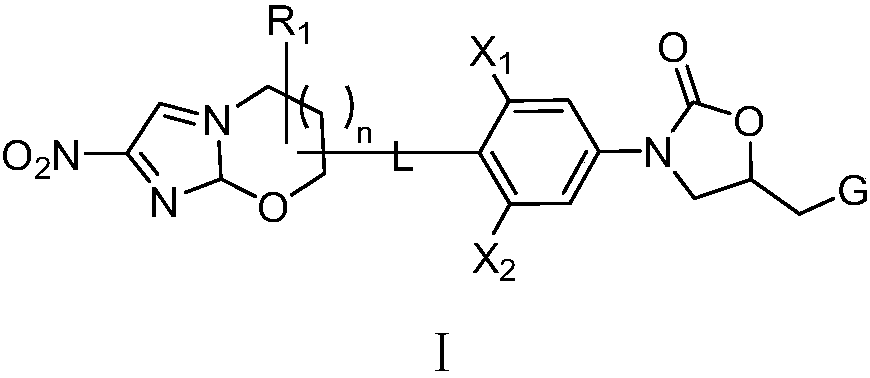
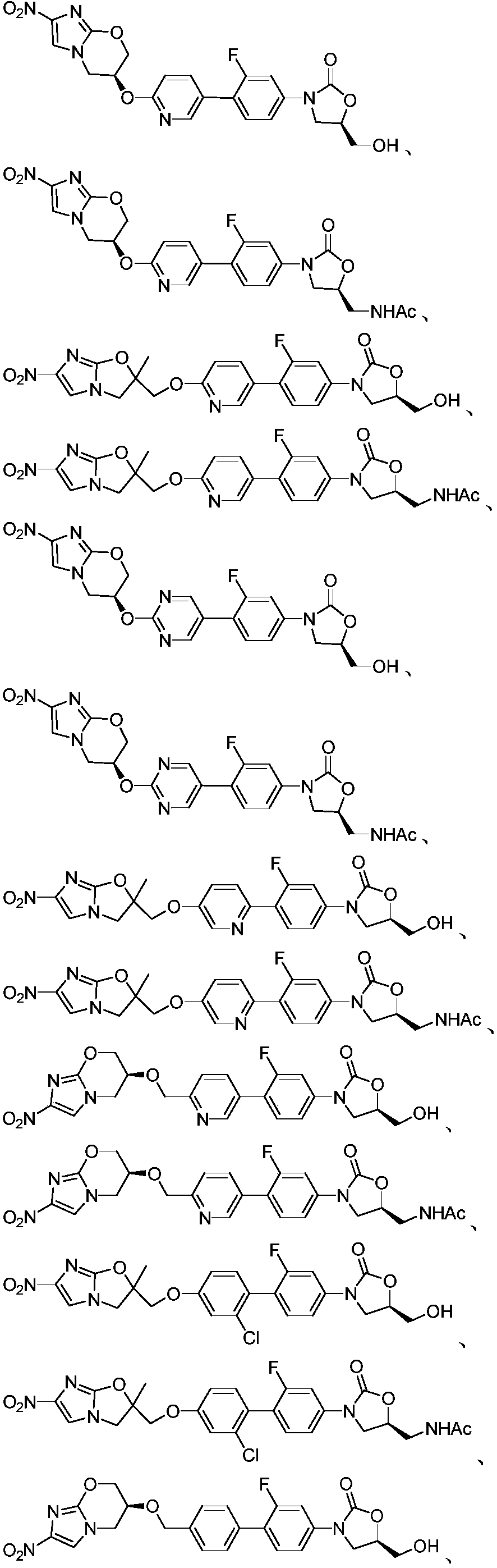
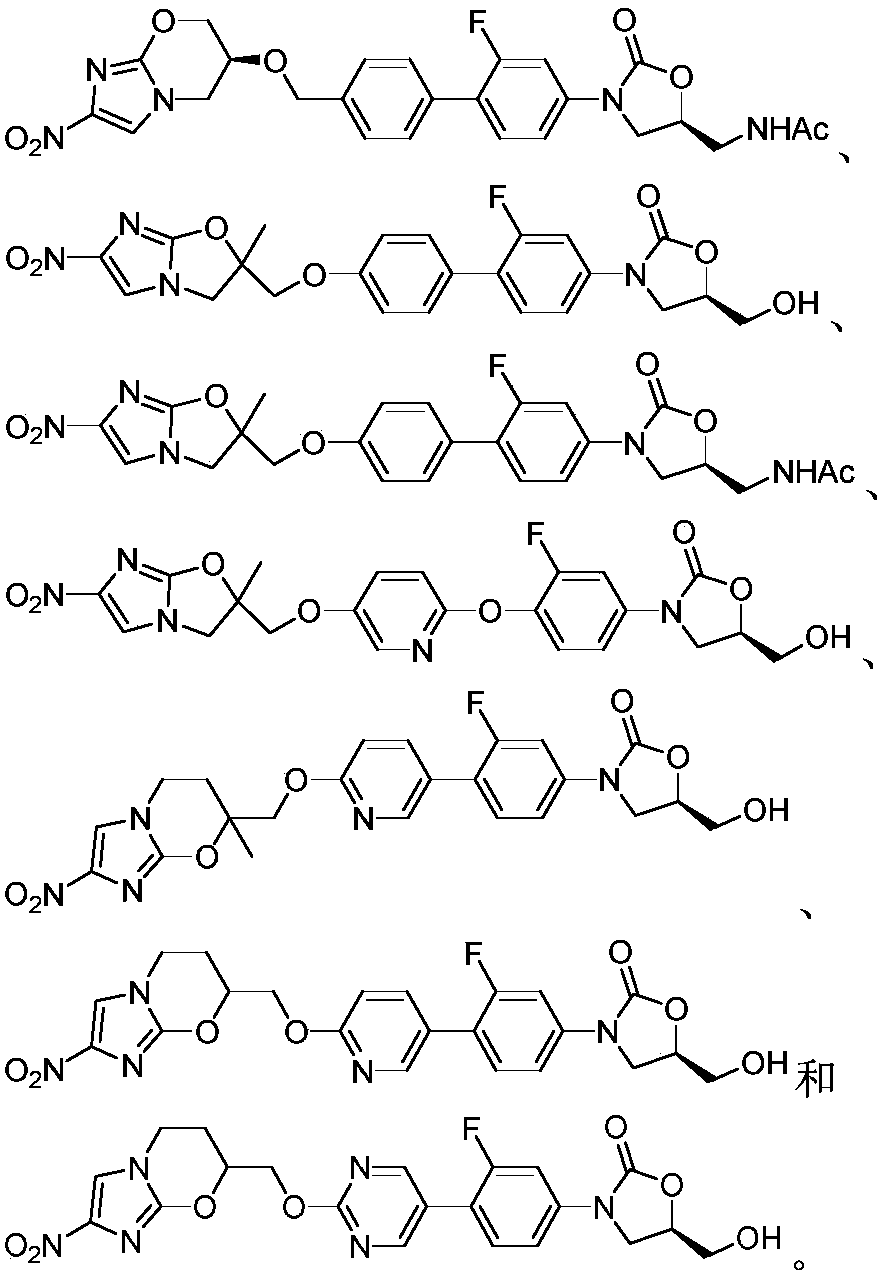
Application of oxazolidone-nitroimidazole coupling molecule
A technology of nitroimidazoles and oxazolidinones, applied in the field of medicine, can solve problems such as lack of metabolic enzyme system, and achieve the effect of treating anaerobic bacterial infectious diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This example provides the application of an oxazolidinone-nitroimidazole coupling molecule in anti-anaerobic Clostridium difficile, and compares and tests the in vitro antibacterial activity of a series of compounds against various bacteria. Methods as below:
[0038] 1. Materials:
[0039] Strains: as shown in Table 2. Strains were obtained from the American Type Culture Collection (ATCC). Store in a -80°C low-temperature refrigerator and resuscitate 2 days before use. Scrape a little frozen bacteria with a sterile inoculation loop, streak and inoculate on a suitable solid medium plate, and put it into a suitable gas culture environment at 35±2°C for 20-48 hours.
[0040] Table 2
[0041] bacterial species
Strain number
Enterococcus faecalis
G+
ATCC 700221
Staphylococcus aureus
G+
ATCC 29213
Klebsiella pneumoniae
G-
ATCC 43816
Acinetobacter baumannii
G-
ATCC 19606
...
Embodiment 2
[0059] This example provides an application of an oxazolidinone-nitroimidazole coupling molecule in anti-anaerobic bacteria, and at the same time tested the antibacterial activity of a series of compounds against anaerobic bacteria in vitro. Methods as below:
[0060] 1. Materials:
[0061] Strains: As shown in Table 4, 14 tested anaerobic bacteria including 13 ATCC strains and one preserved strain of Micromyx are: Bacteroides fragilis, Bifidobacterium longum, Clostridium difficile, Lactobacillus acidophilus, Egger tarda Tetrabacterium, Fusobacterium nucleatum, Gardnerella vaginalis, Animobacterium shy, Peptostreptococcus, Porphyromonas saccharolyticum, Prevotella erus, Propionibacterium acnes, Veillonella bacteria and Helicobacter pylori.
[0062] The test strains were clinically isolated Gram-positive and Gram-negative anaerobic strains deposited at Micromyx. The reference standard strains were obtained from the American Type Culture Collection (ATCC). Inoculate the stra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com