Separation and purification method for recombinant hepatitis B core antigen
A kind of hepatitis B core antigen, separation and purification technology, applied in the field of protein separation and purification, can solve the problem that HBcAg is difficult to meet the pharmaceutical quality standards and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
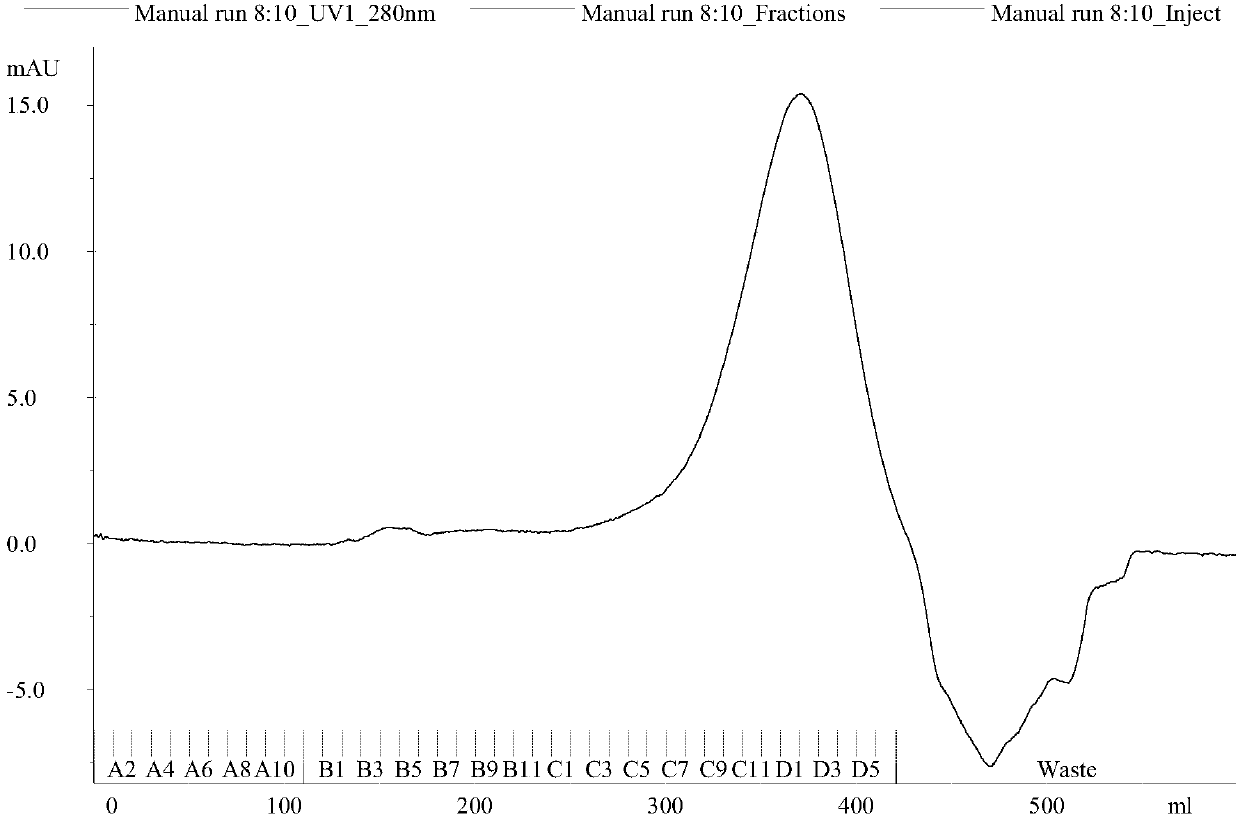
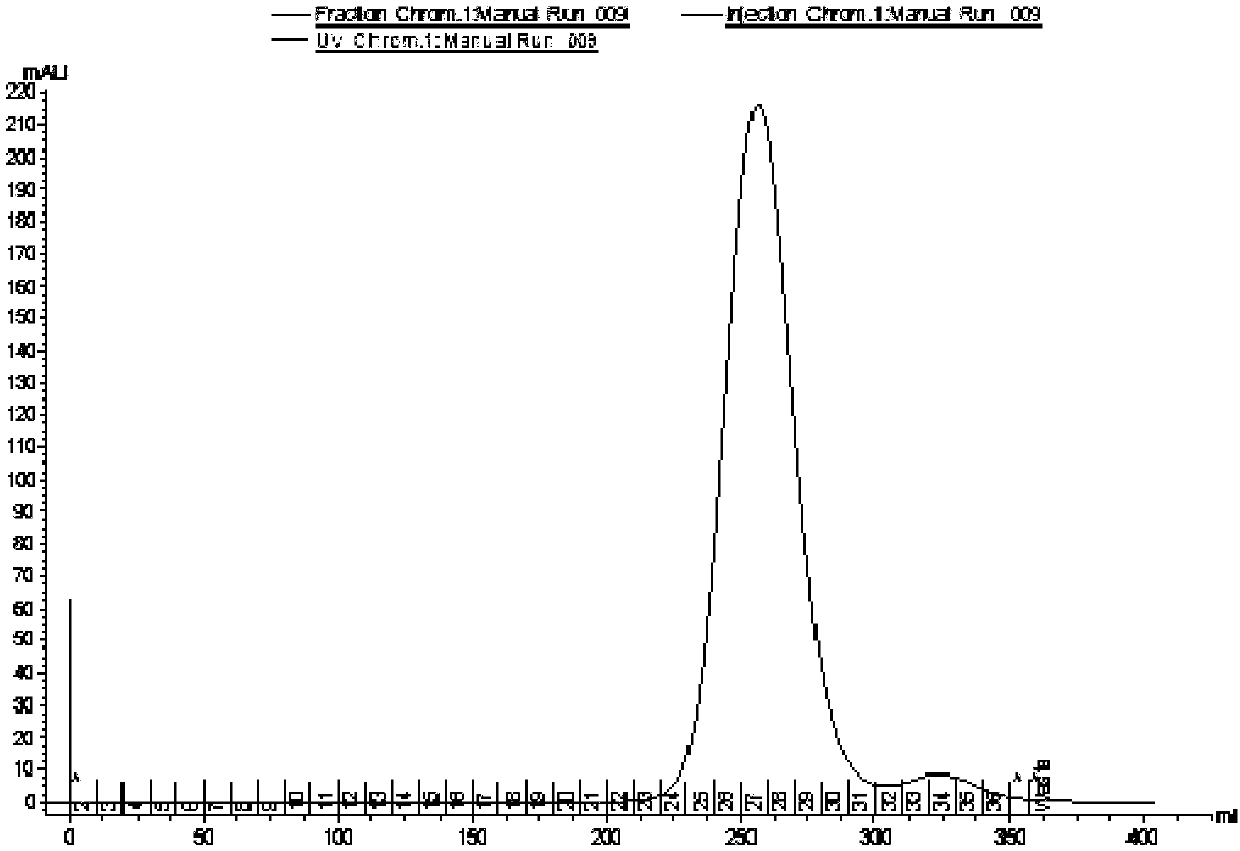
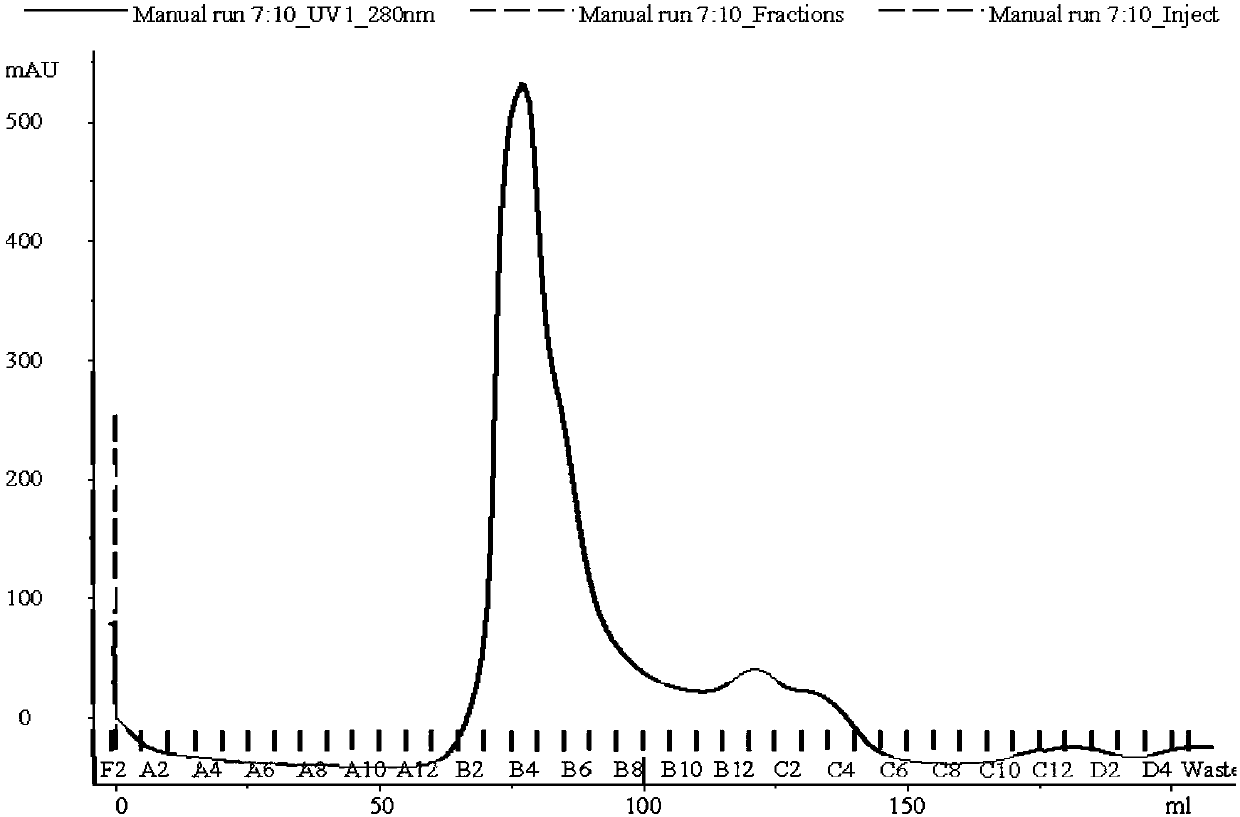
Image
Examples
Embodiment 1
[0071] The purification of embodiment 1 recombinant hepatitis B core antigen
[0072] 1) Bacterial fragmentation
[0073] Take the fermented bacteria, resuspend with 50mM Tris-HCl containing 150mM NaCl, pH 8.0 buffer, maintain the solid content at 10%-30%, obtain the initial bacterial suspension, use a high-pressure homogenizer 1500bar to repeatedly break up The initial bacterial suspension was taken 6 times until the cell disruption rate reached 80-90%; centrifuged at 12000g at 4°C for 30min to remove the precipitate and obtain the supernatant.
[0074] 2) thermal denaturation clarification
[0075] The supernatant obtained in step 1) was placed in a water bath at 60° C. and kept shaking at 50-200 rpm for 0.5-2 hours. Then centrifuge at 12000g, 4°C for 30min, collect the supernatant, and obtain the supernatant;
[0076] 3) Ammonium sulfate precipitation treatment
[0077] Add ammonium sulfate solution dropwise to the supernatant obtained in step 2), the final concentrat...
Embodiment 2
[0090] Example 2 Purification of Recombinant Hepatitis B Core Antigen
[0091] 1) Bacteria fragmentation
[0092] Take the fermented bacteria, resuspend with 50mM Tris-HCl containing 150mM NaCl, pH 8.0 buffer, maintain the solid content at 10%-30%, obtain the initial bacterial suspension, use a high-pressure homogenizer 1500bar to repeatedly break up The initial bacterial suspension was taken 6 times until the cell disruption rate reached 80-90%; centrifuged at 12000g at 4°C for 30min to remove the precipitate and obtain the supernatant.
[0093] 2) thermal denaturation clarification
[0094] The supernatant obtained in step 1) was placed in a water bath at 60° C. and kept shaking at 50-200 rpm for 0.5-2 hours. Then centrifuge at 12000g, 4°C for 30min, collect the supernatant, and obtain the supernatant;
[0095] 3) Ammonium sulfate precipitation treatment
[0096] Add ammonium sulfate solution dropwise to the supernatant obtained in step 2), the final concentration of a...
Embodiment 3
[0109] Example 3 Purification of Recombinant Hepatitis B Core Antigen
[0110] 1) Bacterial fragmentation
[0111] Take the fermented bacteria, resuspend with 50mM Tris-HCl containing 150mM NaCl, pH 8.0 buffer, keep the solid content at 10%-30%, obtain the initial bacterial suspension, use a high-pressure homogenizer 1800bar to repeatedly crush and obtain The initial bacterial suspension was taken 4 times until the cell disruption rate reached 80-90%; centrifuged at 12000g at 4°C for 30min to remove the precipitate to obtain the supernatant.
[0112] 2) thermal denaturation clarification
[0113] The supernatant obtained in step 1) was placed in a water bath at 50° C. and kept shaking at 50-200 rpm for 0.5-2 hours. Then centrifuge at 12000g, 4°C for 30min, collect the supernatant, and obtain the supernatant;
[0114] 3) Ammonium sulfate precipitation treatment
[0115]Add ammonium sulfate solution dropwise to the supernatant obtained in step 2), the final concentration o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com