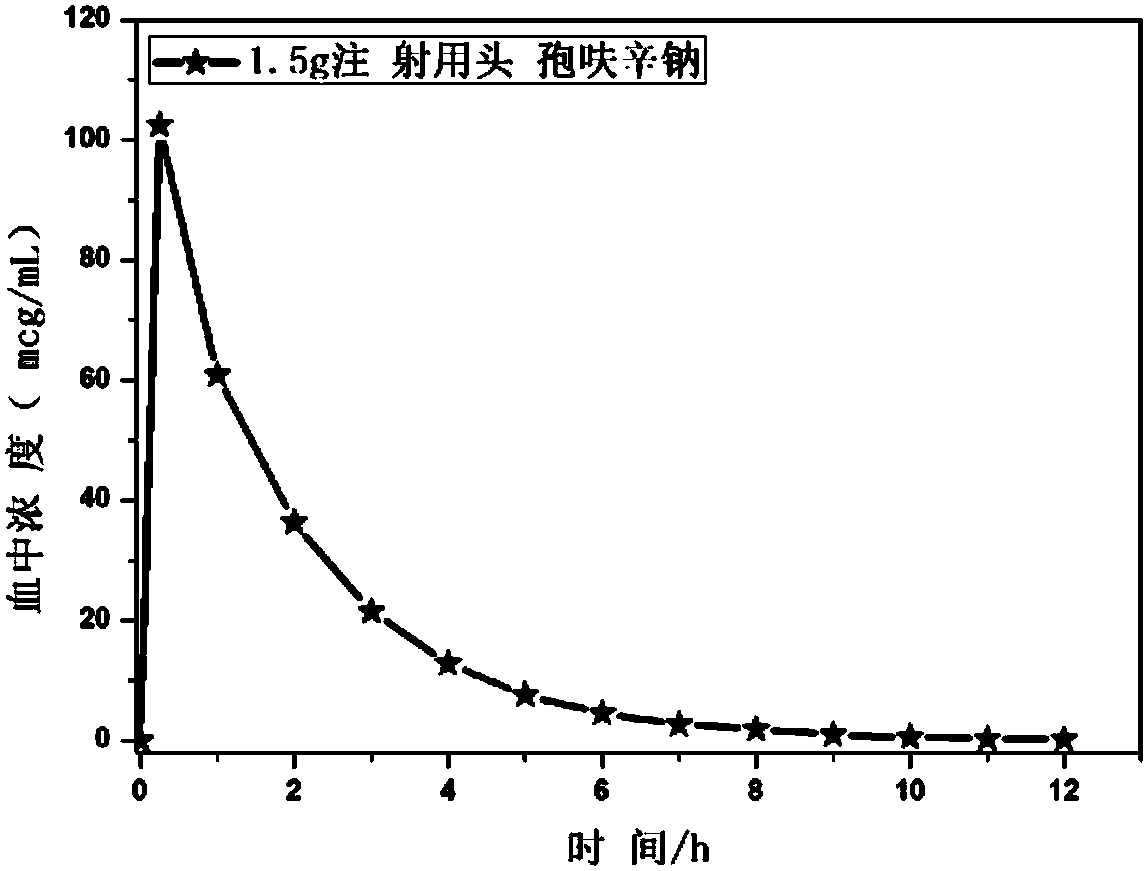
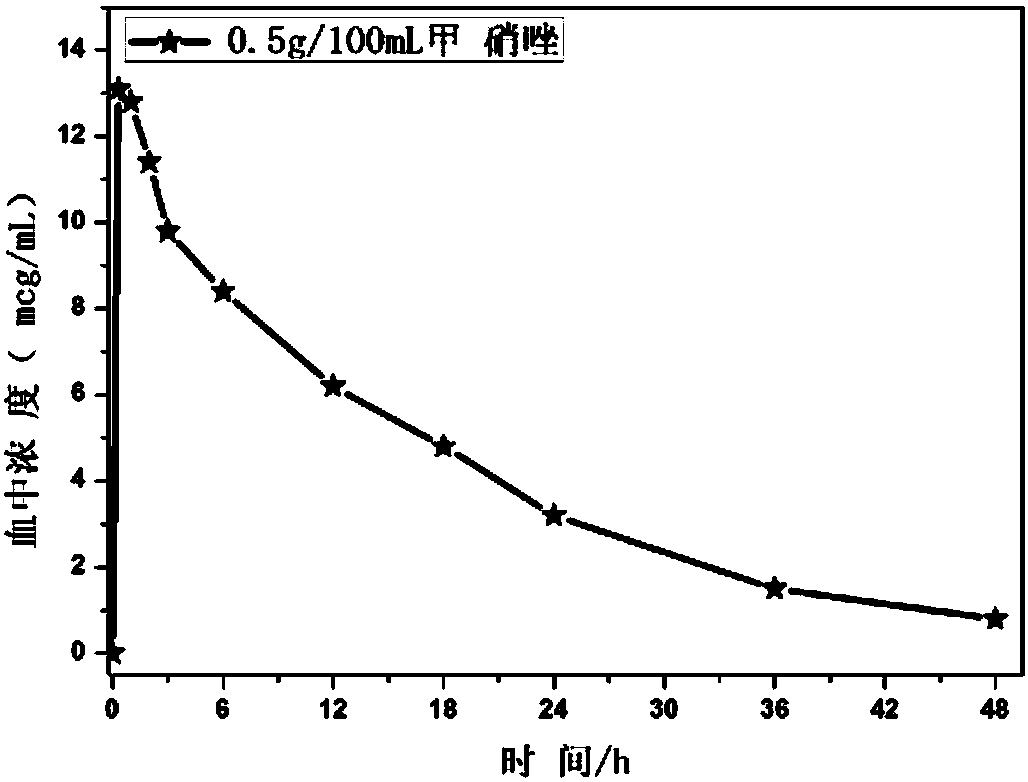
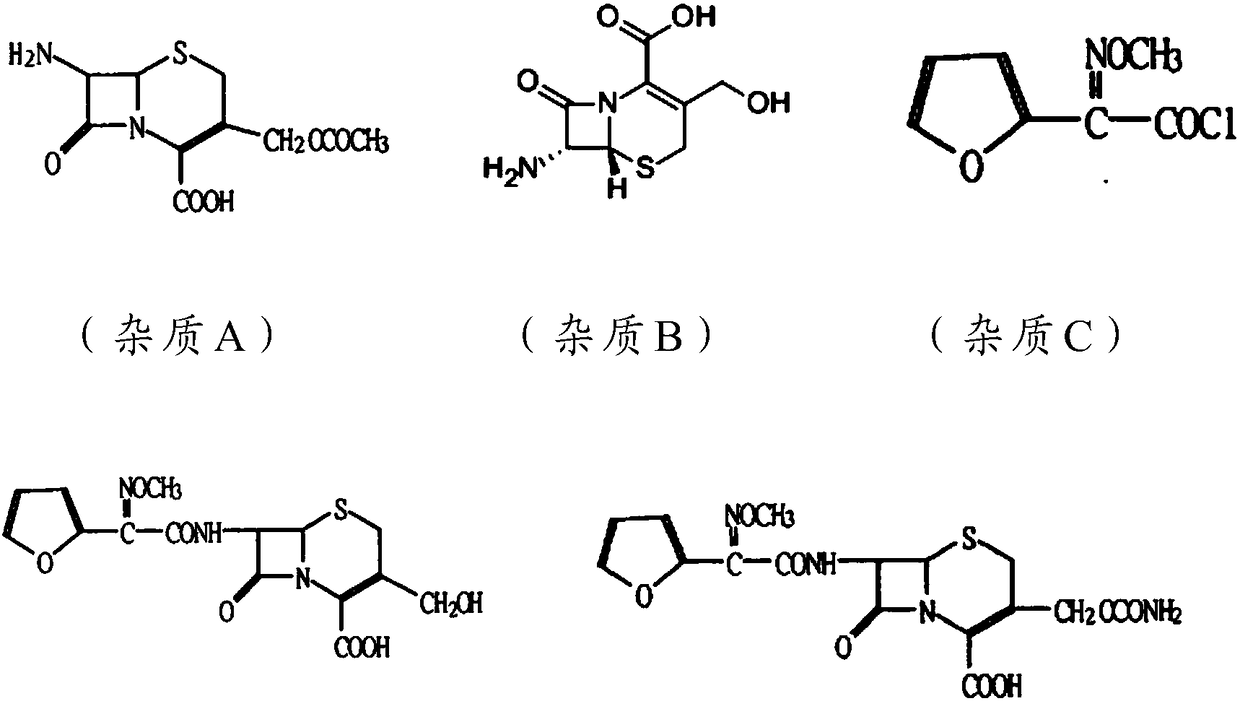
Novel antibiotic composition for preventing and treating aerobic bacterium and anaerobic bacterium mixed infection and preparation method thereof
A technology of mixed infection and composition, which is applied in the field of medicine, can solve the problems of uneven quality, inconsistent control level, and inconsistent curative effect of metronidazole injection, so as to avoid unqualified clarity, improve solution stability, and sterilize the effect Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] The preparation method of described cefuroxime sodium for injection comprises the following steps:
[0060] Step 1: refining the raw material of cefuroxime sodium to reduce remaining process impurities in the synthesis, and controlling the total amount of impurities to be less than 0.5%.
[0061] Since cefuroxime sodium in the present invention is an injection, it directly enters the patient's body through intramuscular injection, intravenous injection or intravenous infusion, so the requirements for its safety are relatively high.
[0062] In the present invention, cefuroxime sodium is refined again to improve the purity of cefuroxime sodium. The water used in the present invention is sterile water for injection.
[0063] In the refining step, cefuroxime sodium is added into the reaction tank, and a mixed solvent is added to dissolve it completely. The cefuroxime sodium includes water, and further, it also contains one of ethanol, acetone, dichloromethane and n-propan...
Embodiment 1
[0178] (1) Preparation of Cefuroxime Sodium for Injection:
[0179] Put 1 kg of cefuroxime sodium bulk drug (Sinopharm Group Zhijun Pharmaceutical Co., Ltd.) in the reaction tank, add acetone: dichloromethane: 8L of mixed solvent with a ratio of 7:2:1 of water for injection, stir and dissolve, add Use 24g of activated carbon for needles, stir for 20min and then filter with a filter membrane of 0.22μm.
[0180] Add 2L of acetone to the solution in portions under stirring, and cool the solution to 5°C at a cooling rate of 5°C / h, grow crystals for 1 hour, filter the precipitate, and dry in vacuum until the water content is 2.5%.
[0181] Put the precipitate in the DECO-PBM-V-2L ball mill of Deco, and carry out aseptic grinding in a 100-level purification environment, wherein the speed is 650r / min, and the grinding time is 15min; nitrogen-filled grinding; all the ground The powder was sieved through a 100 mesh nylon sieve.
[0182] The cefuroxime sodium powder was vacuum-dried a...
experiment example 1
[0196] Get the cefuroxime sodium for injection that makes in embodiment 1, use Karl-Fisher's coulometric titrator to measure the moisture content in the sample, the result is as follows:
[0197]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com