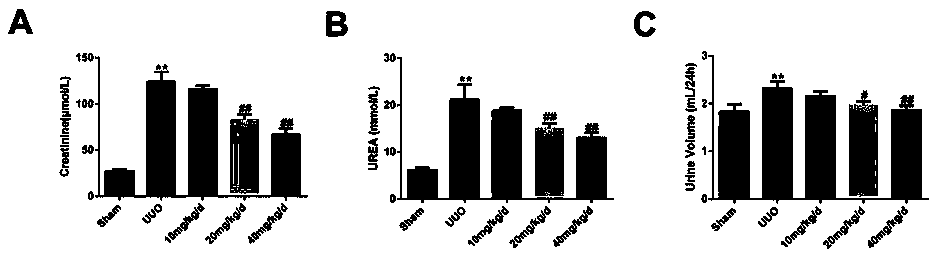
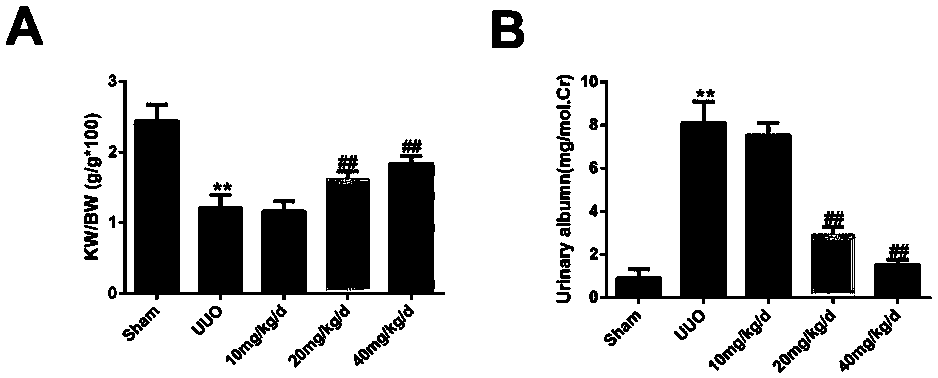
Application of panax japonicus saponin IVa in preparation of drug for treating nephritis or renal fibrosis drug
A technology of ginseng saponins and renal fibrosis, which can be used in the preparation of drug combinations, steroids, and sugar derivatives, etc., and can solve the problems that the gradual progress of chronic kidney disease cannot be completely prevented.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 (preparation of bamboo ginseng saponin Ⅳa)
[0021] Take 20kg of Aralia taibai and add 10 times the volume concentration of 80% ethanol to reflux for extraction 3 times, reflux and extract at 80°C for 60min, concentrate under reduced pressure at 60°C to a relative density of 1.01-1.05 Aralia taibai crude extract; The product was dispersed with distilled water, extracted with chloroform 1:3 (v / v) to remove fat-soluble impurities, extracted 3 times with water-saturated n-butanol (1:3, v / v), combined the 3 extracts, and evaporated with rotary evaporator The instrument was recovered under reduced pressure at 60°C, and evaporated to dryness on a 60°C water bath. The n-butanol extract was separated by silica gel column chromatography, Sephadex LH-20 column chromatography, ODS reverse-phase column chromatography, Lobar column chromatography and reverse-phase HPLC preparation. Using 1D NMR (1H and 13C NMR), 2D NMR (DQCOSY, TOCSY, HMQC, HMBC, NOESY and ROESY) and M...
Embodiment 2
[0022] Embodiment 2 (capsules)
[0023] Weigh 10 mg of bamboo ginseng saponin IVa powder prepared in Example 1, 50-100 mg of starch, 50-70 mg of microcrystalline cellulose, add 70 mg of dextrin, 70 mg of lactose, and 7 mg of sodium carboxymethyl cellulose, and pass through a 60-mesh sieve. Mix evenly, use 85% ethanol as wetting agent to make soft material, pass through a 40-mesh sieve, oven-dry at 60°C for 5 hours, granulate with a 40-mesh sieve, and remove fine powder with a 60-mesh sieve to obtain 40-60-mesh granules. The critical relative humidity of the granules is below 78%, choose No. 1 capsule (0.50 ml) for filling, that is.
Embodiment 3
[0024] Embodiment 3 (tablet)
[0025] Adopt the technology of wet granulation, weigh the bamboo ginsenoside IVa powder prepared in Example 1, the mass ratio of lactose, microcrystalline cellulose, sodium carboxymethyl cellulose and magnesium stearate is: 10:25:83: 6:0.8. Pass the ginseng saponin IVa powder through a 100-mesh sieve, respectively pass through the lactose, microcrystalline cellulose, sodium carboxymethyl cellulose and magnesium stearate through a 60-mesh sieve, and put the obtained uniform powders into clean containers respectively. Soft powder made from 75% ethanol, granulated with a 18-24 mesh sieve, dried at 60°C for 2-4 hours, granulated with a 18-24 mesh sieve, mixed with micronized silica gel and magnesium stearate, pressed into tablets, that is Get a core.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com