Zinc oxide-based photocatalytic material co-doped with niobium nitrogen and its preparation method and application
A catalytic material and co-doping technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of unstable and unobtainable zinc oxide nitrogen doping, and reduce operation Requirements, the effect of simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
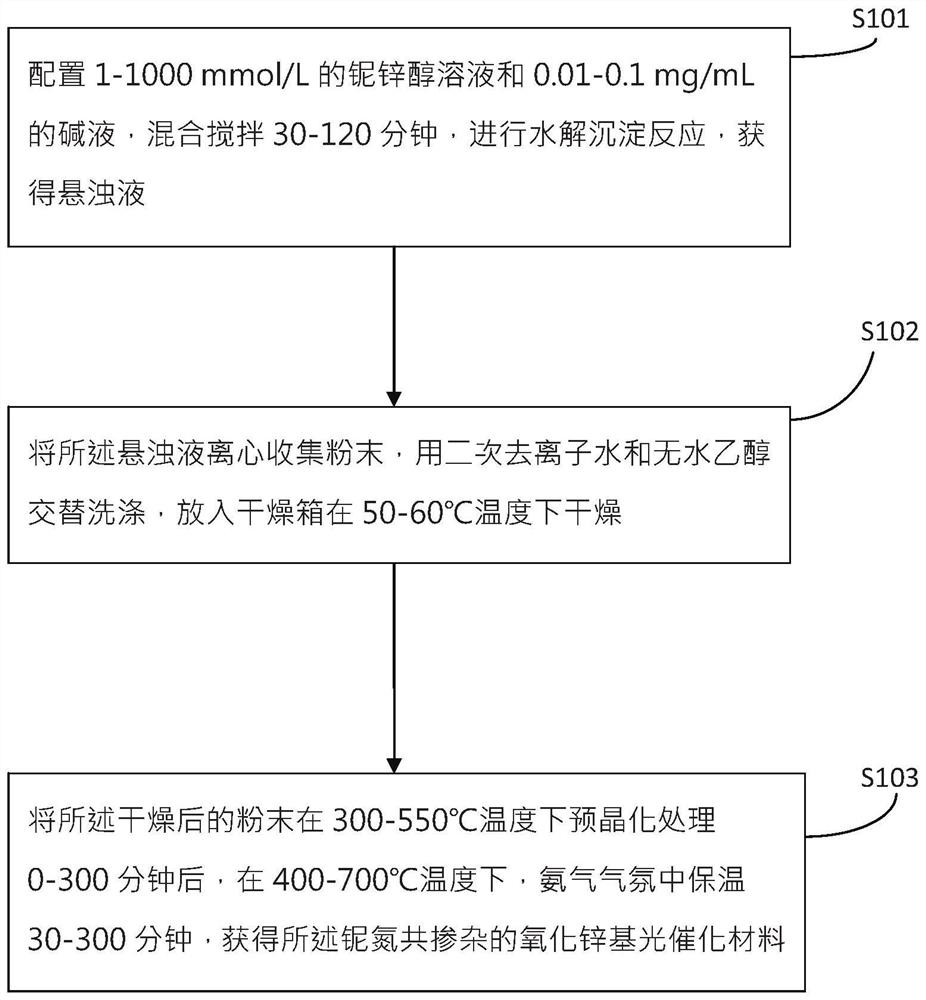
[0023] see figure 1 , the invention provides a kind of preparation method of the zinc oxide base photocatalytic material of niobium nitrogen co-doping, comprises the following steps:
[0024] S101: Prepare 1-1000mmol / L niobium-zinc alcohol solution and 0.01-0.1mg / mL lye, mix and stir for 30-120 minutes, carry out hydrolysis and precipitation reaction, and obtain a suspension;
[0025] S102: Centrifuge the suspension to collect powder, alternately wash with secondary deionized water and absolute ethanol, and put it into a drying oven to dry at a temperature of 50-60°C;
[0026] S103: Pre-crystallize the dried powder at a temperature of 300-550°C for 0-300 minutes, and then heat it for 30-300 minutes at a temperature of 400-700°C in an ammonia atmosphere to obtain the niobium-nitrogen co- Doped ZnO-based photocatalytic materials.
[0027] Further, in step S101, the niobium-zinc alcohol solution is prepared by dissolving zinc chloride and niobium chloride in the alcohol solutio...
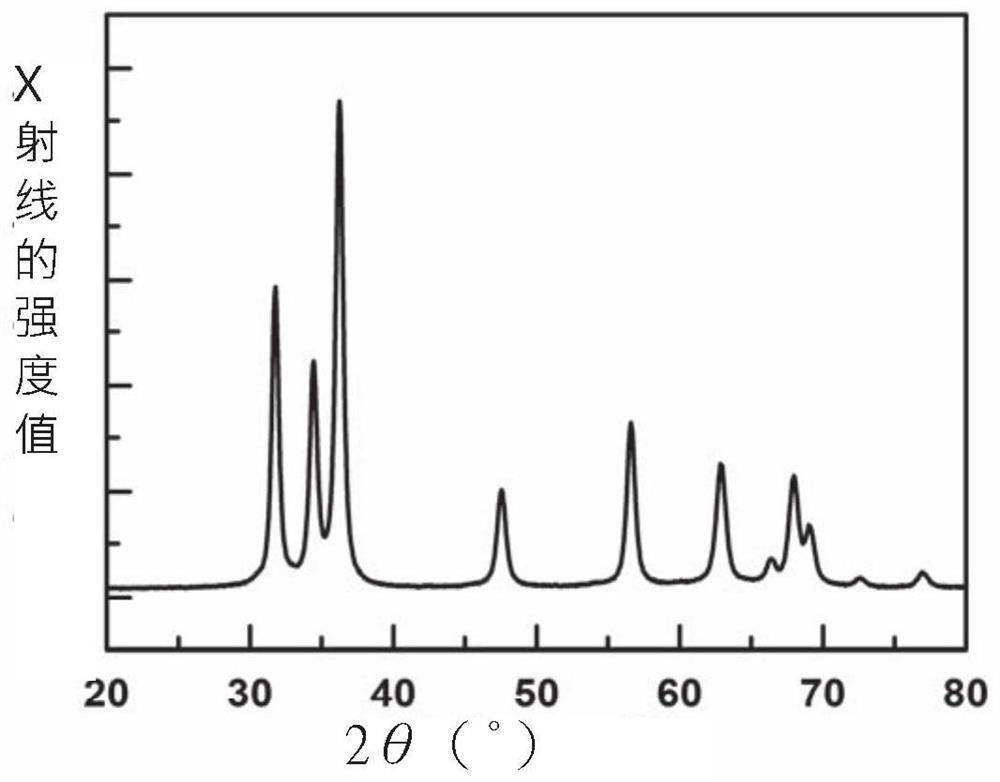
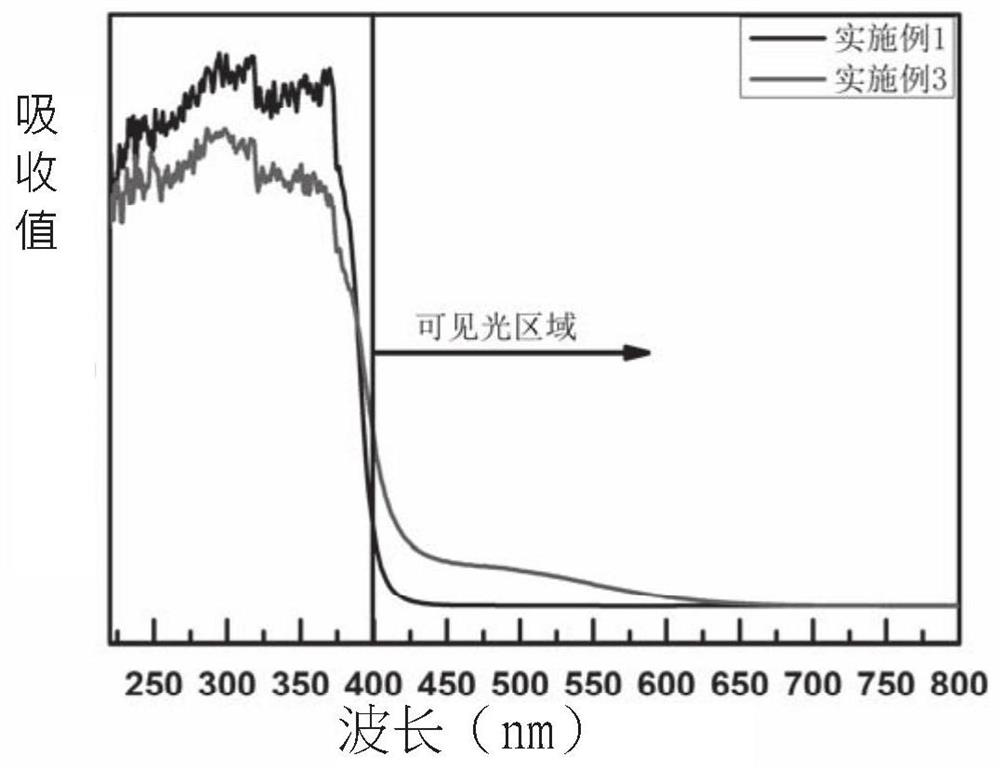
Embodiment 1
[0035] Prepare a niobium-zinc alcohol solution, in which the concentration of zinc ions is 100mmol / L, the concentration of niobium ions is 1mmol / L, and 0.035mg / mL of lye, and the two solutions are mixed and stirred at room temperature for 30min to obtain a suspension;
[0036] The obtained suspension was centrifuged to collect the powder, alternately washed 8 times with secondary deionized water and absolute ethanol, and dried in a drying oven at 60°C for one day;
[0037]The obtained powder was pre-crystallized at 500° C. for 100 minutes, and then kept at 550° C. for 120 minutes in an ammonia atmosphere to obtain the final niobium-nitrogen co-doped zinc oxide-based photocatalytic material.
Embodiment 2
[0039] Prepare a niobium-zinc alcohol solution, in which the concentration of zinc ions is 100mmol / L, the concentration of niobium ions is 2mmol / L, and 0.04mg / mL of lye, and the two solutions are mixed and stirred at room temperature for 30min to obtain a suspension;
[0040] The obtained suspension was centrifuged to collect the powder, alternately washed 8 times with secondary deionized water and absolute ethanol, and dried in a drying oven at 60°C for one day;
[0041] The obtained powder was pre-crystallized at 500° C. for 120 minutes, and then kept at 550° C. for 120 minutes in an ammonia atmosphere to obtain the final niobium-nitrogen co-doped zinc oxide-based photocatalytic material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| lattice constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com