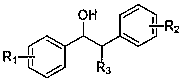
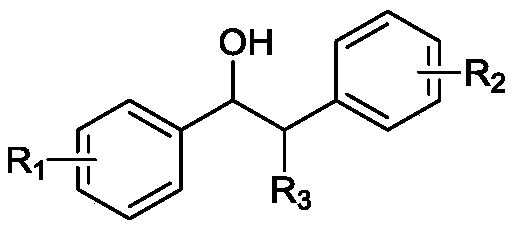
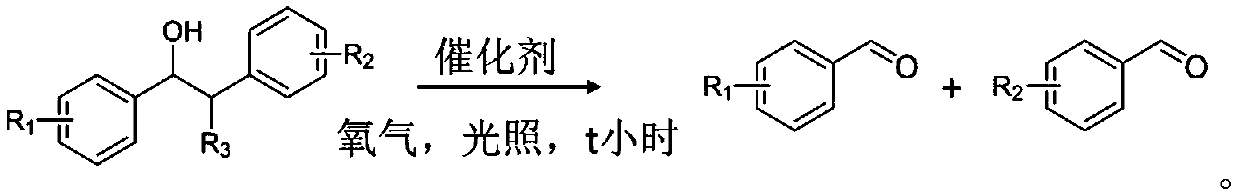
A method for preparing aldehyde compounds by photocatalytic oxidative cleavage of β-hydroxy compound c-c bonds
A technology for hydroxyl compounds and aldehyde compounds is applied in the field of photocatalytic oxidation and cleavage of C-C bonds of β-hydroxy compounds to prepare aldehyde compounds. Highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In a 10mL pressure-resistant photoreaction tube, add 20mg CuO x · CeO 2 (Cu:Ce=1:4, the catalyst is prepared by co-precipitation method), 1mmol benzphenyl ethanol, 5mL acetonitrile as solvent, 1atm oxygen, under room temperature stirring conditions, LED light irradiation for 12 hours, the light intensity is 20mW / cm –2 , after the reaction, the conversion rate detected by GC-MS was 98%, and the selectivity of benzaldehyde was >99%. The catalyst was filtered off, the solvent was removed by rotary evaporation, petroleum ether and ethyl acetate (volume ratio 10:1) were separated on a column, and the separation yield of benzaldehyde could reach 90%.
Embodiment 2
[0043] In a 10mL pressure-resistant photoreaction tube, add 20mg CeO 2 ·TiO 2 (Ce:Ti=1:10, the catalyst is prepared by deposition and precipitation method), 1mmol benzphenyl ethanol, 5mL acetonitrile as solvent, 1atm oxygen, under room temperature stirring conditions, LED light irradiation for 12 hours, the light intensity is 20mW / cm –2, after the reaction, the GC-MS detection conversion rate was 98%, and the selectivity of benzaldehyde was >99%. The catalyst was filtered off, the solvent was removed by rotary evaporation, petroleum ether and ethyl acetate (volume ratio 10:1) column separation, and the separation yield of benzaldehyde could reach 86%.
Embodiment 3
[0045] In a 10mL pressure-resistant reaction tube, add 20mg CuO x · CeO 2 ·TiO 2 (Cu:Ce:Ti=1:4:40, the catalyst is prepared by deposition and precipitation), 1mmol benzphenyl ethanol, 5mL acetonitrile as solvent, 1atm oxygen, under room temperature stirring conditions, LED light irradiation for 12 hours, the light intensity is 20mW / cm –2 , after the reaction, the GC-MS detection conversion rate was 98%, and the selectivity of benzaldehyde was >99%. The catalyst was filtered off, the solvent was removed by rotary evaporation, petroleum ether and ethyl acetate (volume ratio 10:1) were separated on a column, and the separation yield of benzaldehyde could reach 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com