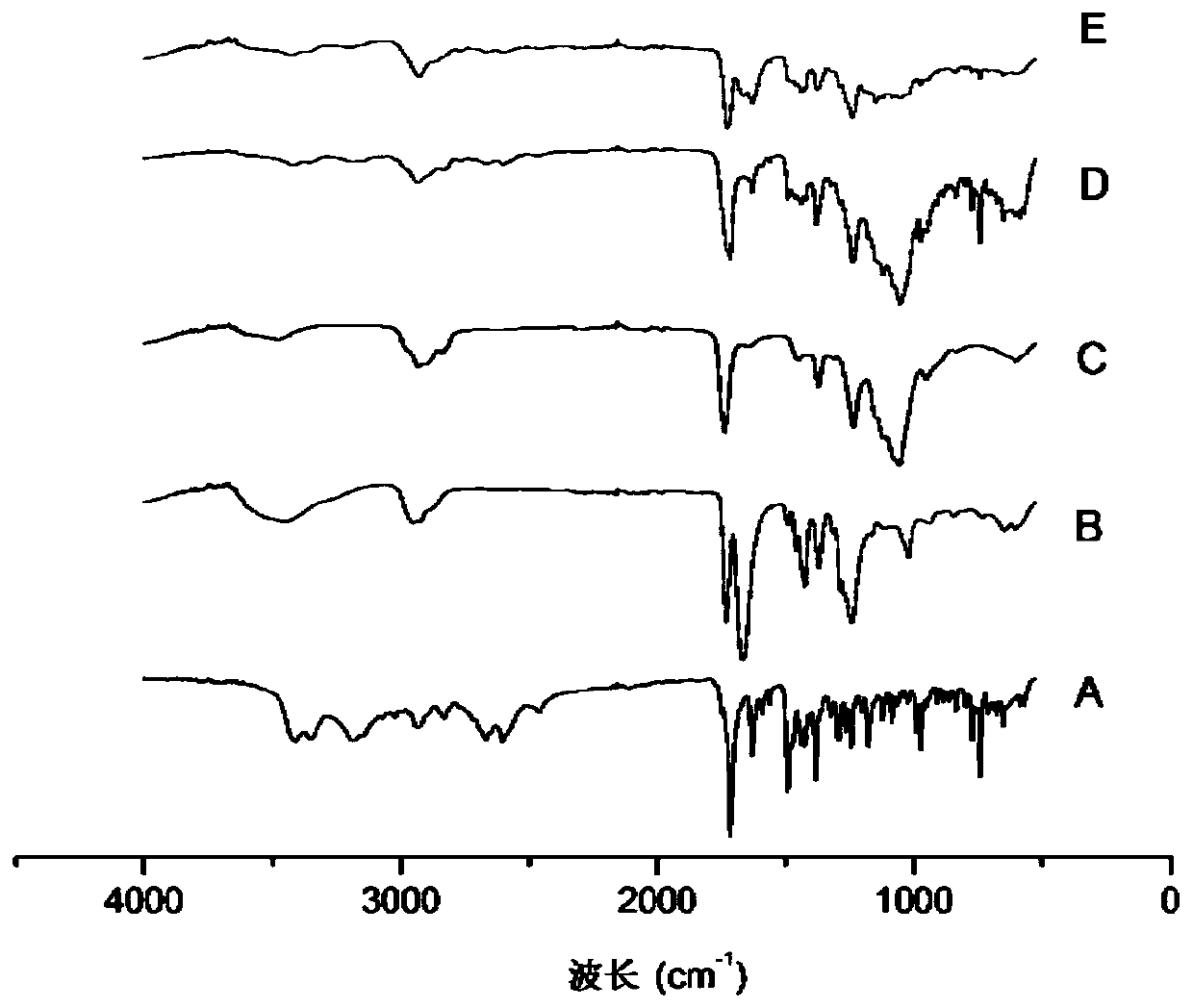
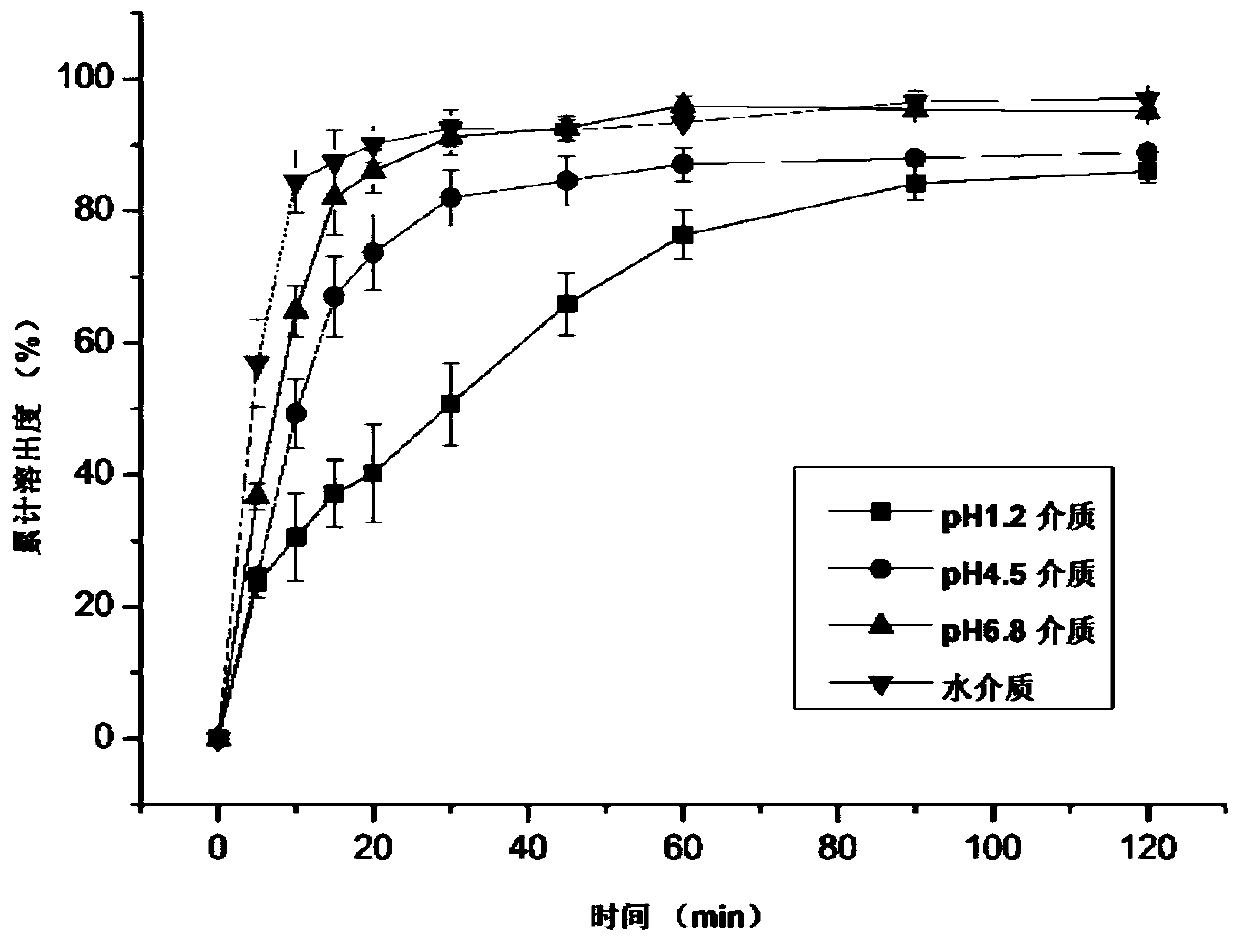
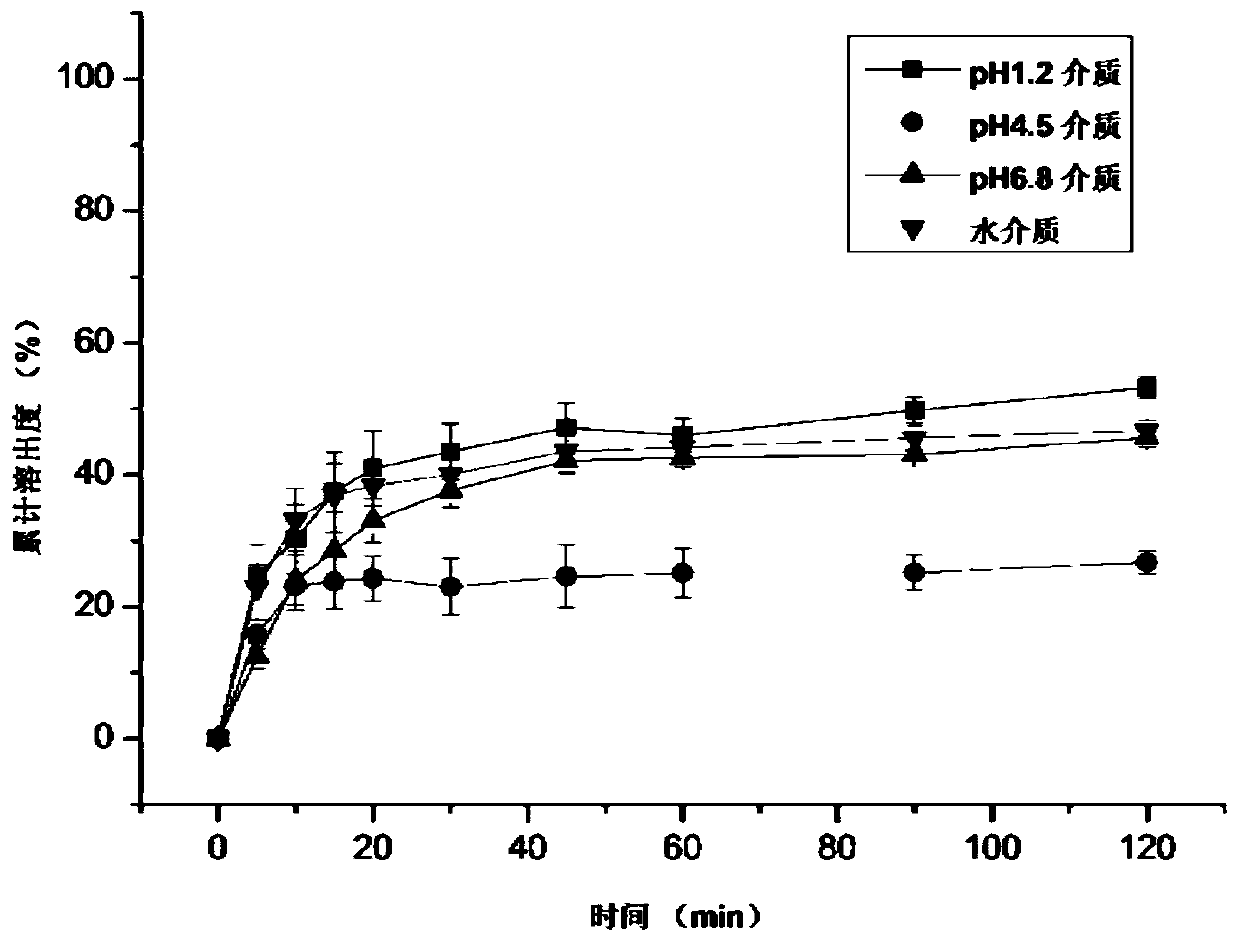
A kind of ziprasidone hydrochloride solid dispersible tablet and hot-melt extrusion method thereof
A technology of hot-melt extrusion of ziprasidone hydrochloride, which is applied in the direction of pharmaceutical formulations, organic active ingredients, and medical preparations of non-effective ingredients, etc. It can solve the problems of limited application range, high experiment cost, and large production input volume, etc. problem, to achieve the effect of improved performance in vivo and in vitro, good reproducibility, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] prescription:
[0053]
[0054] Preparation method: After fully mixing ziprasidone hydrochloride, CopovidoneS630, EudragitEPO, Soluplus, extrude strips through a twin-screw hot-melt extruder, after cooling and solidifying, crush them for 90 seconds to obtain a solid dispersion, and then add Filler microcrystalline cellulose, disintegrant croscarmellose sodium and lubricant magnesium stearate, after mixing again, use a single-punch tablet machine to directly compress the powder into tablets, and the weight of the tablet is 3-4kg Within 1-2min, the disintegration time limit is sufficient.
Embodiment 2
[0056] prescription:
[0057]
[0058] Preparation method: After fully mixing ziprasidone hydrochloride, CopovidoneS630, EudragitEPO, Soluplus, extrude strips through a twin-screw hot-melt extruder, after cooling and solidifying, crush them for 90 seconds to obtain a solid dispersion, and then add Filler microcrystalline cellulose, disintegrant croscarmellose sodium and lubricant magnesium stearate, after mixing again, use a single-punch tablet machine to directly compress the powder into tablets, and the weight of the tablet is 3-4kg Within 1-2min, the disintegration time limit is sufficient.
Embodiment 3
[0060] prescription:
[0061]
[0062] Preparation method: After fully mixing ziprasidone hydrochloride, CopovidoneS630, EudragitEPO, Soluplus, extrude strips through a twin-screw hot-melt extruder, after cooling and solidifying, crush them for 90 seconds to obtain a solid dispersion, and then add Filler microcrystalline cellulose, disintegrant croscarmellose sodium and lubricant magnesium stearate, after mixing again, use a single-punch tablet machine to directly compress the powder into tablets, and the weight of the tablet is 3-4kg Within 1-2min, the disintegration time limit is sufficient.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com