Stable polypeptide used for resisting estrogenic receptor alpha and application thereof
A technology of fluorescein and fluorescein isothiocyanate, which is applied in the direction of peptides, antineoplastic drugs, peptide/protein components, etc., can solve problems such as poor effects, and achieve significant technological progress, high resistance to degradation, and stable helicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] In one embodiment of the invention, stabilized polypeptides based on non-natural aspartic acid to form amide bond closures are able to cross cell membranes.
[0100] The present invention is based on the sequence HKILHRLLQ on the coactivator that interacts with estrogen receptor α, and uses the polypeptide solid-phase synthesis method to synthesize the polypeptide. The linear polypeptide is named as polypeptide 1. At the same time, the i-position lysine and i+3-position histidine sites that do not participate in the interaction are mutated into amino acids with side chain carboxyl groups (here, take unnatural aspartic acid as an example) and amino acids with side chain amino groups (Here, diaminopropionic acid is taken as an example), the polypeptide is synthesized in solid phase, the crude polypeptide is cut from the solid phase resin with trifluoroacetic acid, and the target polypeptide is purified by high performance liquid chromatography, and the polypeptide is ident...
Embodiment 2
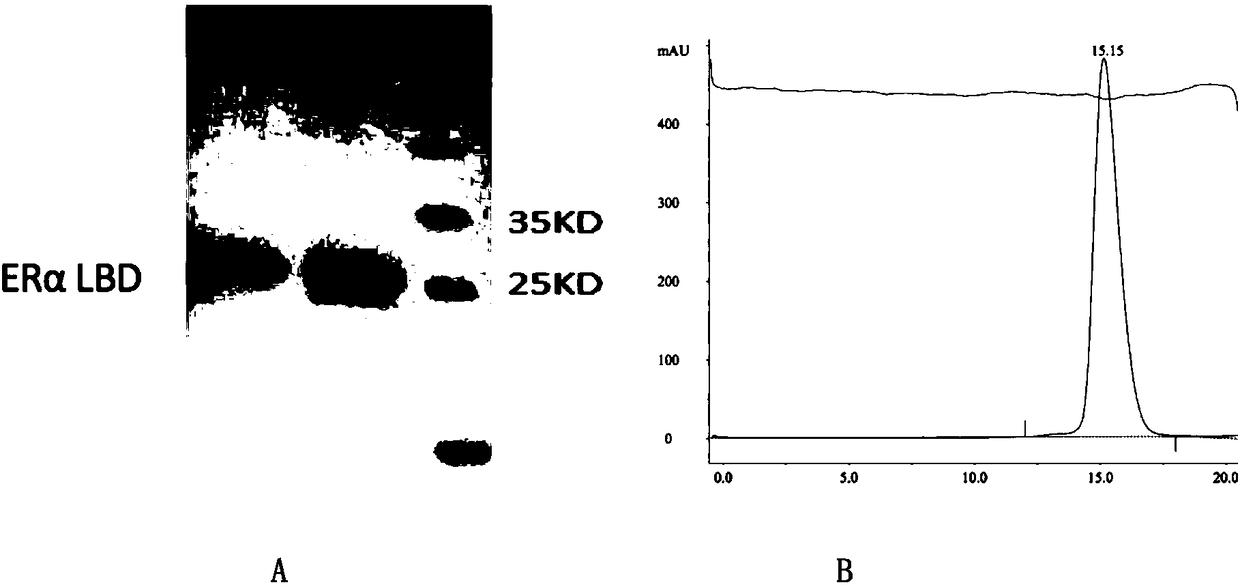
[0107] In order to further understand the present invention, research polypeptide 2 (sequence is H-Arg-cyclo(isoAsp-Ile-Leu-Dap)-Arg-Leu-Leu-Gln-NH 2 ) interacting with the estrogen receptor ERα, and the crystal structure of the complex was resolved. First, a stable polypeptide was synthesized by solid-phase synthesis, and the protein ERα-LBD was purified and separated. The SDS-PAGE electrophoresis and gel chromatography chromatograms of the protein are shown in figure 1 . Then, 2 to 4 times the polypeptide was incubated with ERα-LBD, concentrated to about 4 mg / ml, and then crystals of the complex were obtained by the sessile drop method ( figure 2 ), and then collected multiple sets of X-ray diffraction data, and determined the phase with the molecular replacement method, corrected the structural model with the COOT software, and optimized the structure with the Phenix software, and finally obtained the crystal structure of the protein-stabilized polypeptide complex.
Embodiment 3
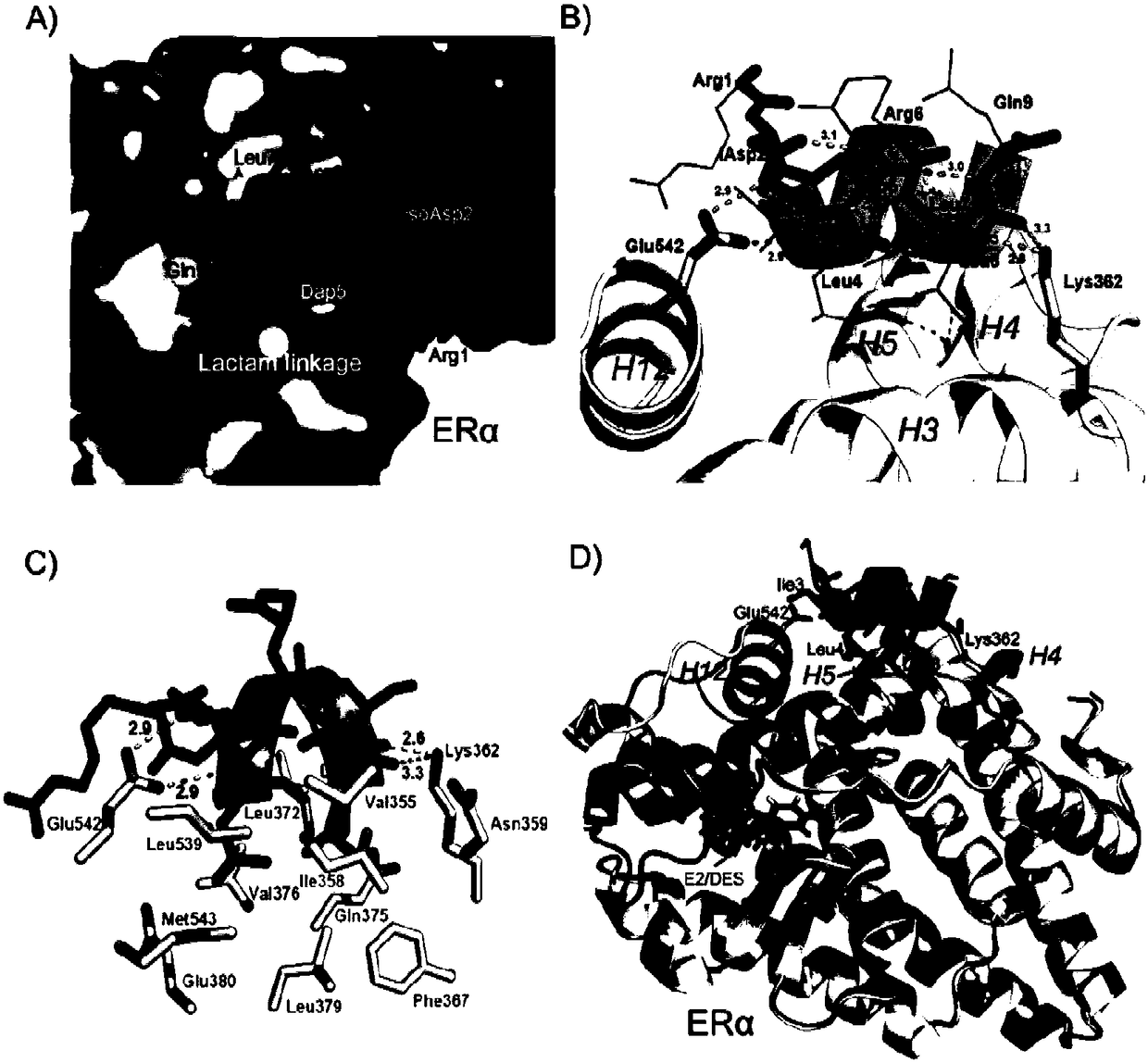
[0109] Crystal structure analysis of the complex of ERα ligand-binding domain and polypeptide 2, see image 3 , it can be seen from the crystal structure diagram that the hydrophobic leucine residue of the stabilizing polypeptide is just inserted into the hydrophobic pocket bound by the coactivator of the ligand binding domain of ERα. The 542-position negatively charged amino acid glutamic acid (Glu) on the surface of the estrogen receptor ERα and the 362-position positively charged amino acid lysine (Lys) form a "charged clamp" structure to further stabilize the binding of the polypeptide by forming an ionic bond with the main chain of the polypeptide. Isoleucine at position 3 and leucine at position 4 can form a hydrogen bond with glutamic acid at position 542 of the ERα ligand binding domain; lysine at position 362 of the ligand binding domain of ERα can form a hydrogen bond with the 7 Leucine at position 8 forms a hydrogen bond, further stabilizing the interaction. The st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com