Preparation method of alkaline anion exchange membrane fuel cell membrane electrode
An anion exchange membrane, alkaline anion technology, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as less impact on performance, and achieve the effects of improving performance, improving pore structure, and alleviating mass transfer polarization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
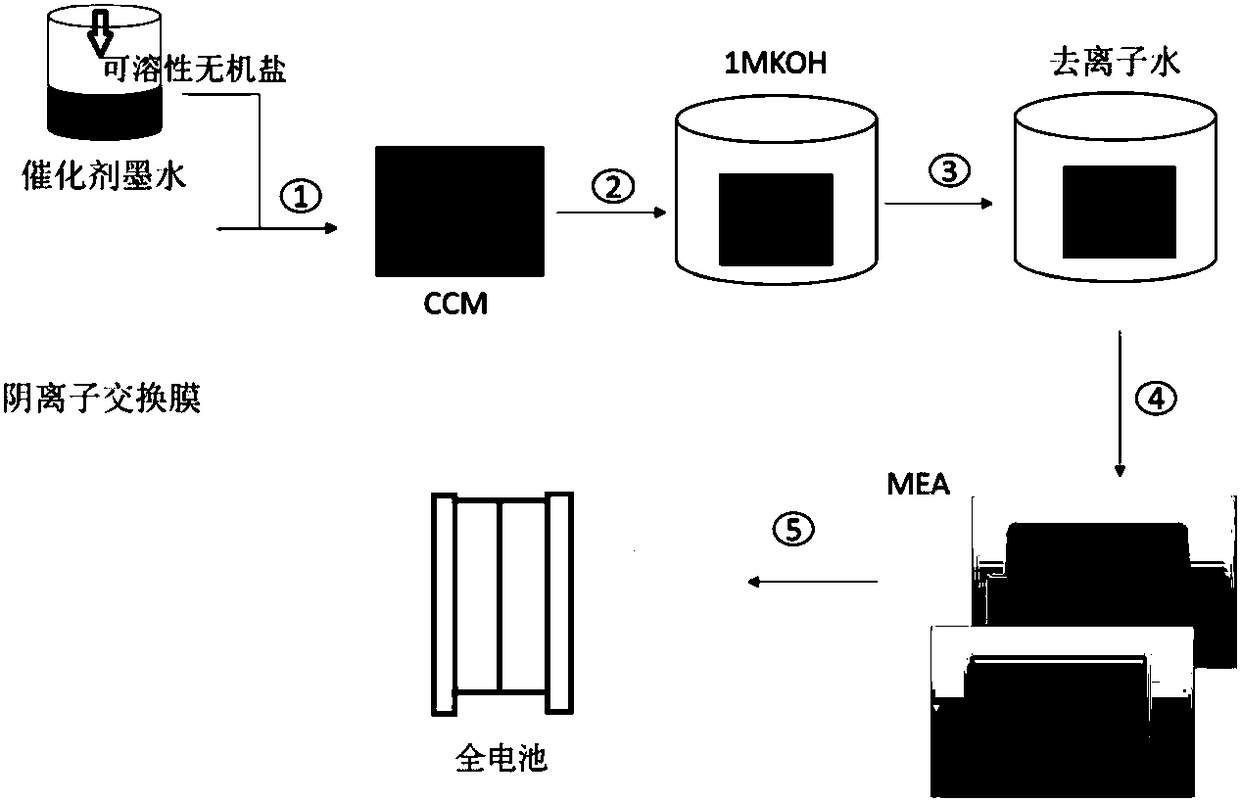
[0026] The membrane electrode preparation method is as follows:
[0027] Select the self-made alkaline anion exchange membrane 24cm in the group 2 .
[0028] Weigh 20.6 mg of 70% Pt / C catalyst, add 1030 microliters of n-propanol to ultrasonically disperse for 30 minutes, and then add the self-made TEA-SEBS-Cl type anion exchange resin solution to form the mass of electrocatalyst and anion exchange resin Catalyst ink with a ratio of 4:1 was ultrasonically dispersed for 60 minutes to make a catalyst slurry.
[0029] On a hot stage at 60°C, the catalyst layer slurry was sprayed on the surface of the basic anion exchange membrane, and after the solvent was completely evaporated, it was naturally cooled to obtain a catalyst-coated membrane electrode.
[0030] Put the above-mentioned catalyst-coated membrane electrode into 1 mole per liter of potassium hydroxide solution, soak for 24 hours, take it out after completion, wash away the surface lye with deionized water, and finally p...
Embodiment 2
[0036] Weigh 20.6 mg of 70% Pt / C catalyst, add 1030 microliters of n-propanol to ultrasonically disperse for 30 minutes, then add 10.3 mg of ammonium oxalate to form a catalyst ink with a mass ratio of inorganic salt and catalyst of 1:2, and ultrasonically disperse 60 minutes.
[0037] Then, the self-made TEA-SEBS-Cl type anion exchange resin solution was added to the above slurry to form a catalyst ink with a mass ratio of electrocatalyst and anion exchange resin of 4:1, which was ultrasonically dispersed for 60 minutes.
[0038] On a hot stage at 60°C, the catalyst layer slurry was sprayed on the surface of the basic anion exchange membrane, and after the solvent was completely evaporated, it was naturally cooled to obtain a catalyst-coated membrane electrode.
[0039] Put the above-mentioned catalyst-coated membrane electrode into 1 mole per liter of potassium hydroxide solution, soak for 24 hours, take it out after completion, wash away the surface lye with deionized water...
Embodiment 3
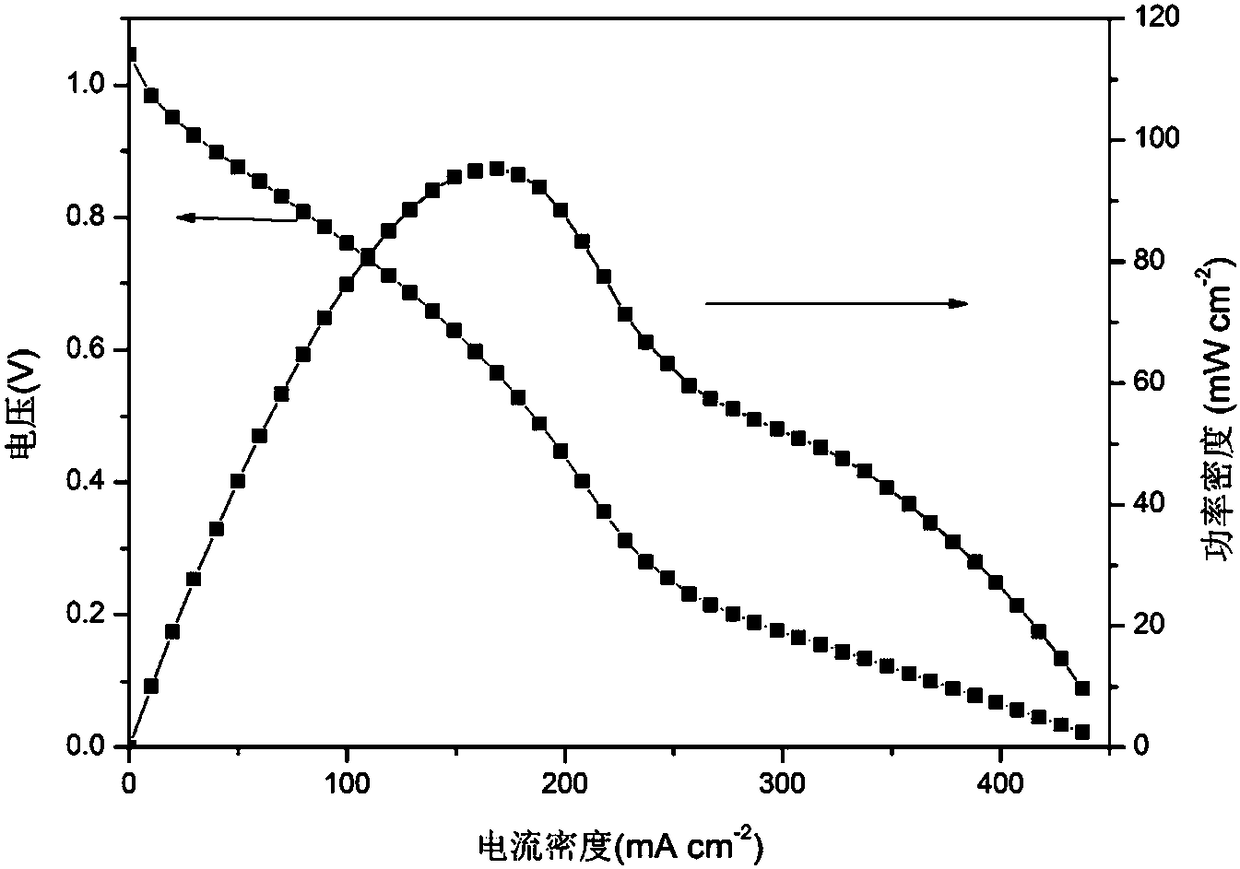
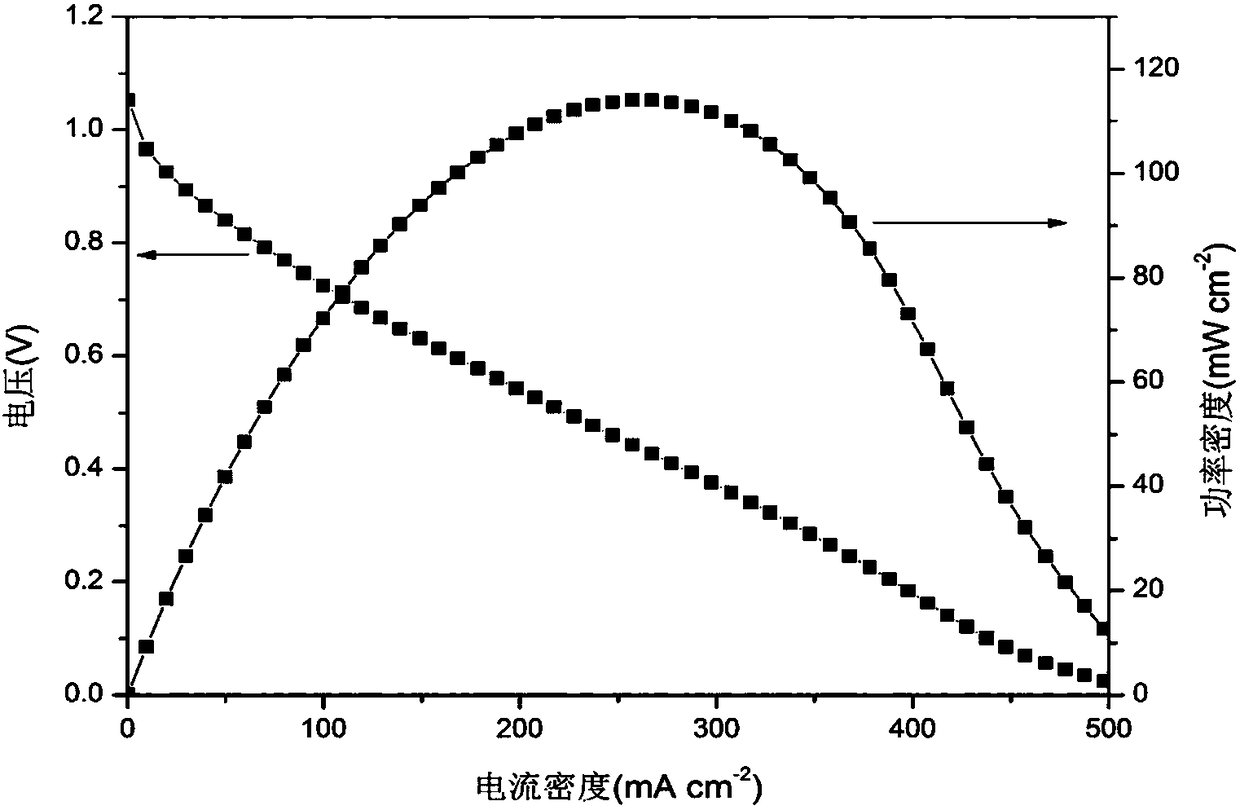
[0045]The difference from Example 1 is that the mass ratio of the inorganic salt to the catalyst is 1:1. The average pore diameter of the catalytic layer is 19.87 microns, and the specific pore volume is 1.85 cm 3 / g. Assemble the battery, the polarization curve and power density curve of the battery are as follows Figure 4 As shown, its peak power density is 98mW / cm 2 , at a current density of 208mA / cm 2 The maximum power is obtained at the position, compared with the electrode prepared by the traditional method (peak power density 95.3mW / cm 2 ), its peak power density increased by 2.7mW / cm 2 . at 250mA / cm 2 , the voltage attenuation is 0.37V, which is 0.13V slower than the traditional method.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Specific pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com