Nanofiber membrane containing calcitonin lipidosome and preparation method and application thereof
A nanofibrous membrane and liposome technology, which is applied in liposome delivery, medical preparations containing active ingredients, fiber treatment, etc., can solve the problems of poor liposome stability and achieve low Zeta potential and excellent stability , The effect of expanding the application space
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A kind of preparation method of the nanofibrous membrane containing calcitonin liposome of the present embodiment, concrete steps are as follows:
[0038](1) Weigh 150 mg of soybean lecithin and 25 mg of cholesterol, add them into a mixed solvent of ether-methanol (8:1, v / v), and rotatively evaporate in a constant temperature water bath at 35°C until the organic solvent is completely removed, until A uniform lipid film forms on the wall. Then, 4 ml of salmon calcitonin phosphate buffer solution was added to the obtained lipid film, immersed in a constant temperature water bath at 70° C. for 10 seconds, vortexed and eluted, and repeated 3 times to obtain a suspension. The resulting suspension was incubated in a water bath at 10°C for 1 hour, then the probe was sonicated for 3 minutes in an ice-water bath (200W ultrasonic power, 3 seconds of ultrasound, 3 seconds off), and passed through a 0.22 μm microporous membrane to obtain calcitonin-loaded nanolipids. body. Slowly...
Embodiment 2
[0046] Compared with Example 1, the preparation method of a nanofibrous membrane containing calcitonin liposomes in this example is different in that the mass fraction of sodium alginate aqueous solution is 2%, and the rest of the steps and parameters are exactly the same.
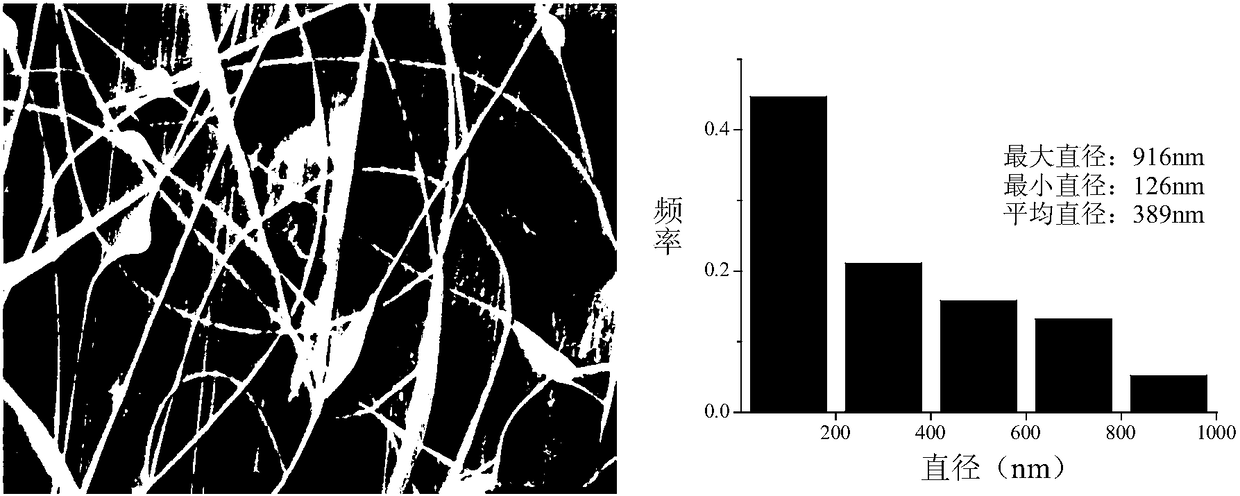
[0047] The SEM figure and fiber diameter distribution of the nanofibrous membrane containing calcitonin liposome finally obtained in the present embodiment are as follows: image 3 shown.
Embodiment 3
[0049] Compared with Example 1, the preparation method of a nanofibrous membrane containing calcitonin liposomes in this example is different in that the mass fraction of sodium alginate aqueous solution is 3%, and the rest of the steps and parameters are exactly the same.
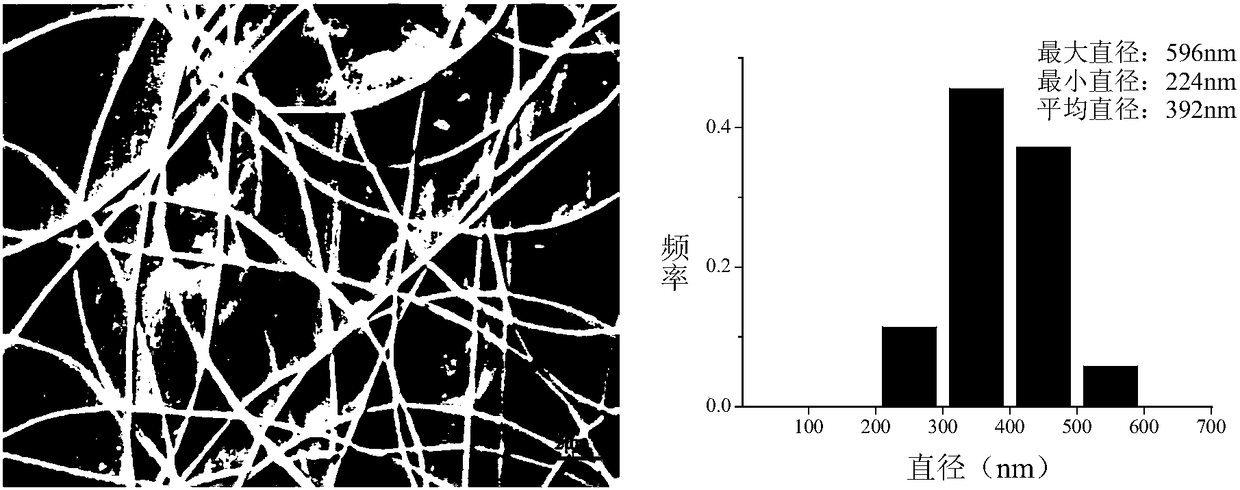
[0050] The SEM figure and fiber diameter distribution of the nanofibrous membrane containing calcitonin liposome finally obtained in the present embodiment are as follows: Figure 4 shown.
[0051] One, to the investigation of the release rate of the nanofibrous membrane containing calcitonin liposomes obtained by the invention under the in vitro simulated gastrointestinal tract environment, the specific implementation steps are as follows:
[0052] Refer to the second appendix of the Chinese Pharmacopoeia 2010 edition, the second method (slurry method) of in vitro release test method and make appropriate modifications, the specific conditions are: temperature 37±0.5°C, release medium is simulated artifici...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com