Eco-friendly general preparation method for pyridyl porphyrin metallization
A technology of pyridyl porphyrin and tetrapyridyl porphyrin, which is applied in the field of green general preparation of pyridyl porphyrin metallization, can solve the problems of unfavorable metal porphyrin material application, high toxicity, complicated purification, etc., and improve the synthesis efficiency And late application efficiency, low equipment requirements, and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A green general preparation method for metallation of pyridyl porphyrins, comprising the following steps:
[0029] (1) Prepare a standard concentration of 1 M HCl solution based on concentrated hydrochloric acid;
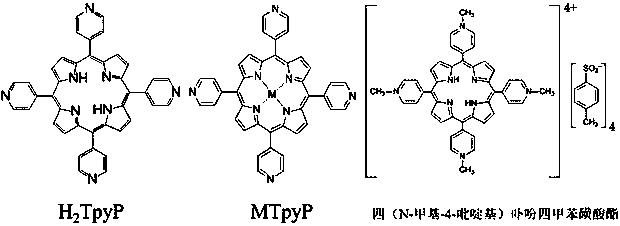
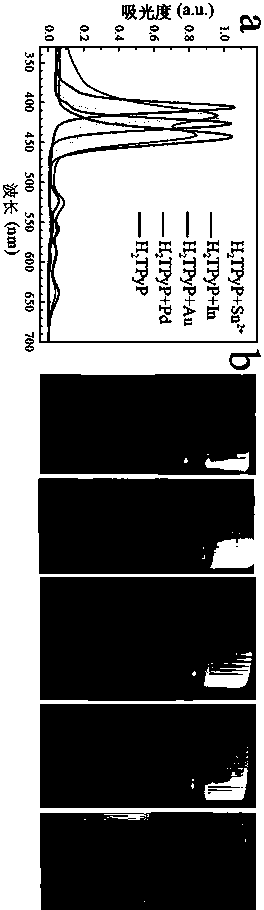
[0030] (2) Dissolve H with the HCl solution in (1) 2 TPyP powder, formulated H 2 TPyP / HCl solution, making H 2 H in TPyP / HCl solution 2 TpyP concentration is 0.01 M, HCl concentration is 0.2 M;
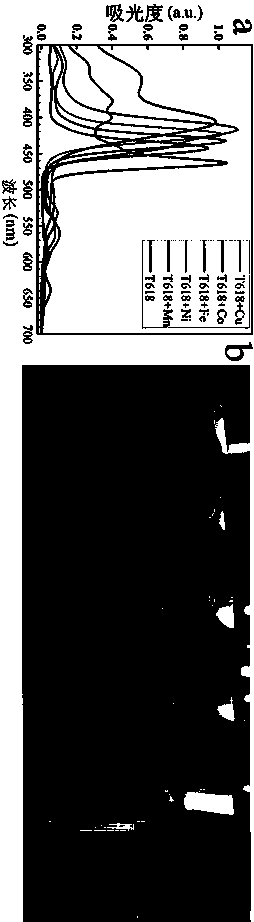
[0031] (3) will H 2 TPyP / HCl solution and different kinds of metal salts: cobalt nitrate hexahydrate Co(NO 3 ) 2 ·6H 2 O; indium nitrate tetrahydrate In(NO 3 ) 3 4H 2 O; manganese acetate tetrahydrate (C 4 h 6 MnO 4 4H 2 O) were mixed at a molar ratio of 1:1.2. Without introducing any other organic solvents, they were put into a reaction bottle and placed in an oven at 80°C for 12 h to complete metalloporphyrins CoTPyP, InTPyP, Preparation of MnTPyP;
[0032] (4) Take the reacted solution in (3) out of the oven, cool to room temperature and add 1 M Na...
Embodiment 2
[0034] A kind of green general preparation method of pyridyl porphyrin metallation, differs from Example 1 in that in step (3) H 2 TPyP / HCl solution and metal salt copper nitrate trihydrate Cu(NO 3 ) 2 ·3H 2 O, stannous chloride dihydrate SnCl 2 2H 2 O was mixed at a molar ratio of 1:1.1. Without introducing any other organic solvents, the preparation of different metalloporphyrins was completed at room temperature for 3 h, and CuTPyP and SnTPyP were prepared respectively.
Embodiment 3
[0036] A kind of green general preparation method of pyridyl porphyrin metallation, differs from Example 1 in that in step (3) H 2 TPyP / HCl solution and metal salt nickel nitrate hexahydrate Ni(NO 3 ) 2 ·6H 2 O, iron nitrate nonahydrate Fe(NO 3 ) 3 9H 2 O was mixed at a molar ratio of 1:2, and NiTPyP and FeTPyP were prepared respectively in a reactor at 180 °C for 24 h without introducing any other organic solvents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com