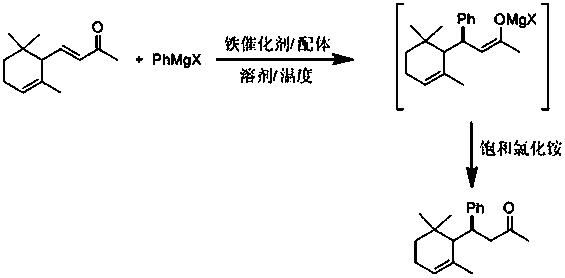
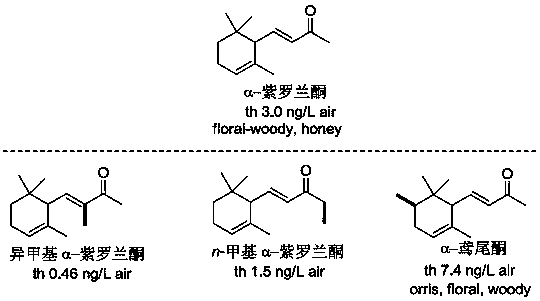
Novel synthetic spice based on alpha-ionone and preparation method of novel synthetic spice
A technology for ionone and synthetic fragrance, which is applied in the field of new synthetic fragrance based on ɑ-ionone and its preparation, and achieves the effects of environmental friendliness, high regio- and stereoselectivity, and convenient industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
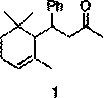
[0025] Add ferric acetylacetonate (0.02 mmol) and ligand hexamethylphosphoric triamide (0.05 mmol) into the reaction tube, and add solvent toluene (5 ml) and α-ionone ( 0.5 mmol), placed in a temperature of 0°C, a solution of phenylmagnesium bromide (1 mmol) in ether was added dropwise to the reaction tube under stirring, and the reaction was continued at 0°C. After 4 hours, gas chromatography detected that the reaction was complete, and the reaction tube was returned to room temperature and cooled to zero, and saturated ammonium chloride solution was added dropwise to quench the reaction, and water was added after returning to room temperature. Extract with ether, wash the organic phase with 1% hydrochloric acid, saturated sodium bicarbonate and saturated brine successively, then dry with anhydrous sodium sulfate, filter, concentrate, and flash column chromatography to obtain 4-(2,6,6 - Trimethyl-2-cyclohexen-1-yl)-5-methyl-hexan-2-one, 75% yield, the product is a colorless l...
Embodiment 2
[0027] Add ferric chloride (0.02 mmol) and ligand triphenylphosphine (0.2 mmol) into the reaction tube, and add solvent diethyl ether (5 ml) and α-ionone (1 mmol ), placed at a temperature of -10 degrees, and added dropwise a solution of phenylmagnesium chloride (1 mmol) in tetrahydrofuran to the reaction tube under stirring, and kept at -10 degrees to continue the reaction. After 10 hours, gas chromatography detected that the reaction was complete, and the reaction tube was returned to room temperature and cooled to zero, and saturated ammonium chloride solution was added dropwise to quench the reaction, and water was added after returning to room temperature. Extract with ether, wash the organic phase with 1% hydrochloric acid, saturated sodium bicarbonate and saturated brine successively, then dry with anhydrous sodium sulfate, filter, concentrate, and flash column chromatography to obtain 4-(2,6,6 - Trimethyl-2-cyclohexen-1-yl)-5-hexen-2-one, yield 57%, product is colorles...
Embodiment 3
[0029] Add ferric chloride hexahydrate (0.02 mmol) and ligand trimethylchlorosilane (0.3 mmol) into the reaction tube, and add solvent tetrahydrofuran (15 ml) and α-ionone ( 1 mmol), placed at a temperature of 25 degrees, and a THF solution of phenylmagnesium bromide (1 mmol) was added dropwise to the reaction tube under stirring, and the reaction was continued at 25 degrees. After 2 hours, gas chromatography detected that the reaction was complete, and the reaction tube was returned to room temperature and cooled to zero, and saturated ammonium chloride solution was added dropwise to quench the reaction, and water was added after returning to room temperature. Extract with ether, wash the organic phase with 1% hydrochloric acid, saturated sodium bicarbonate and saturated brine successively, then dry with anhydrous sodium sulfate, filter, concentrate, and flash column chromatography to obtain 4-(2,6,6 - Trimethyl-2-cyclohexen-1-yl)-4-phenyl-hexan-2-one, 50% yield, the product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com