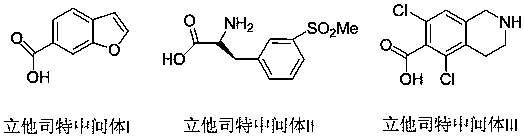
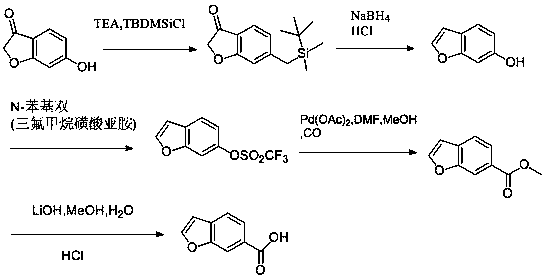
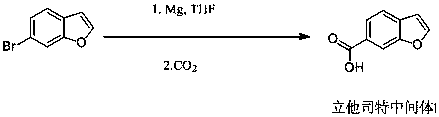Synthesis method of lifitegrast intermediate
A technology of intermediates and ster, which is applied in the field of medicinal chemistry, can solve the problems of unfavorable industrial production, difficult control of Grignard reaction, and low reaction yield, and achieve the effects of simplified reaction steps, mild reaction conditions and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Preparation of 6-methyl formate benzofuran:
[0032] Add 5.28g (0.0268mol) of 6-bromobenzofuran into a 250mL three-necked flask, add 80ml of dry tetrahydrofuran, stir to dissolve, put it in a freezer and cool it down to -65°C, add dropwise n-butyllithium (2M ) 14.74mL, keep the temperature below -60°C during the dropwise addition process, the dropwise addition is completed in 1h, continue to react for 3 hours, under this reaction condition, add 2.89g (0.0321mol) of dimethyl carbonate dropwise After the addition is complete, control the reaction temperature to -65°C. After 4 hours of reaction, the reaction is complete. Add saturated ammonium chloride solution dropwise at low temperature to quench the reaction. After evaporating the solvent under reduced pressure, add 100 mL of water and 100 mL of dichloromethane to stir After layering, the organic layer was washed twice with saturated brine, 100 mL each time, the dichloromethane layer was dried with anhydrous magnesiu...
Embodiment 2
[0036] 1) Preparation of 6-methyl formate benzofuran:
[0037] Add 4.55g (0.0231mol) of 6-bromobenzofuran into a 250mL three-neck flask, add 80ml of dry tetrahydrofuran, stir to dissolve, put it in a freezer and cool it down to -65°C, add n-butyllithium (2M ) 15mL, keep the temperature below -60°C during the dropwise addition process, the dropwise addition is completed in 1h, continue to react for 3 hours, under this reaction condition, add 2.28g (0.0254mol) of dimethyl carbonate dropwise After completion, control the reaction temperature to -65°C. After 4 hours of reaction, the reaction is complete. Add saturated ammonium chloride solution dropwise at low temperature to quench the reaction. After evaporating the solvent under reduced pressure, add 100mL of water and 100mL of methylene chloride to dissolve After layering, the organic layer was washed twice with saturated brine, 100 mL each time, the dichloromethane layer was dried with anhydrous magnesium sulfate and filtered,...
Embodiment 3
[0041] 1) Preparation of 6-methyl formate benzofuran:
[0042]Add 8.28g (0.042mol) of 6-bromobenzofuran into a 250mL three-neck flask, add 100ml of dry tetrahydrofuran, stir to dissolve, put it in a freezer and cool it down to -65°C, add dropwise n-butyllithium (2M ) 23.1mL, keep the temperature below -60°C during the dropwise addition process, the dropwise addition is completed in 1h, continue to react for 3 hours, under this reaction condition, add 4.9g (0.054mol) of dimethyl carbonate dropwise After the addition is complete, control the reaction temperature to -65°C. After 4 hours of reaction, the reaction is complete. Add saturated ammonium chloride solution dropwise at low temperature to quench the reaction. After evaporating the solvent under reduced pressure, add 120 mL of water and 120 mL of dichloromethane to stir After layering, the organic layer was washed twice with saturated brine, 100 mL each time, the dichloromethane layer was dried with anhydrous magnesium sulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com