Caffeate derivative and preparation method thereof
A technology of caffeic acid and its derivatives, which is applied in the field of medicine, can solve the problems that affect the clinical application of caffeic acid, it is not easy to absorb and utilize, and the polarity of caffeic acid is high, and achieve excellent bioavailability, stable yield, and good biological activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
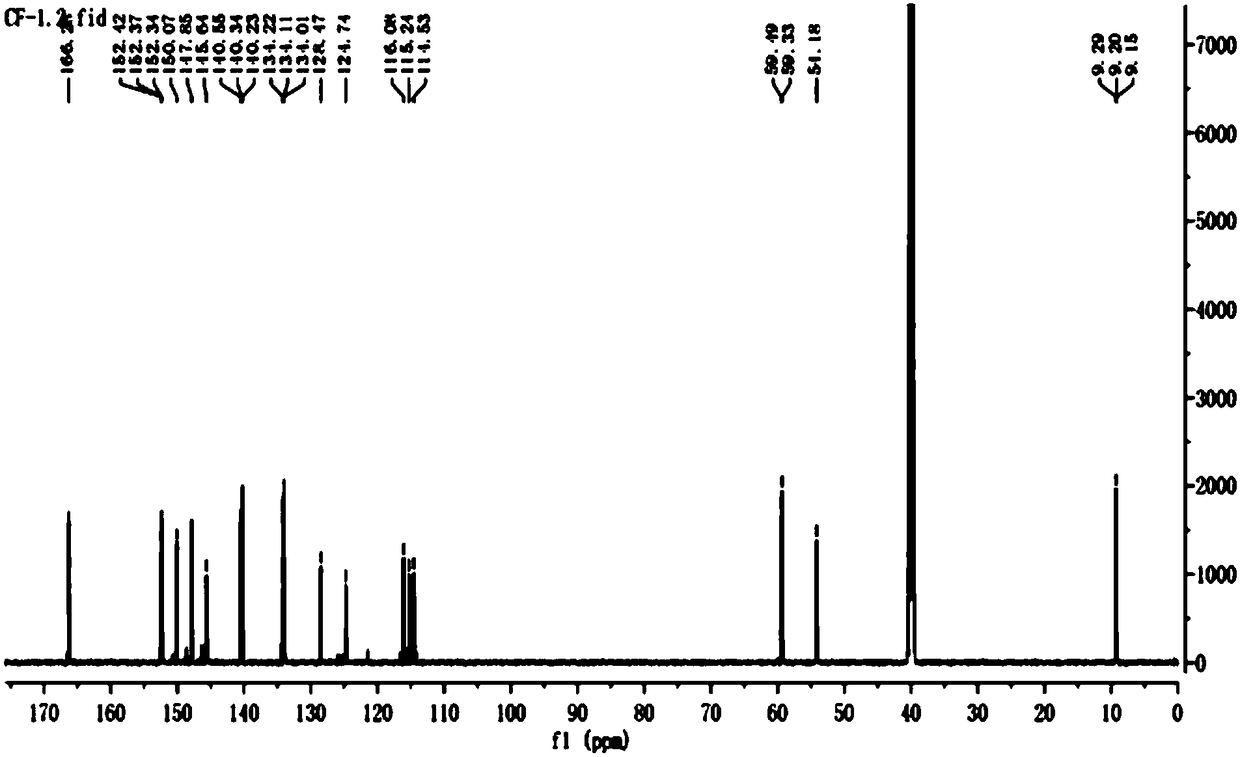
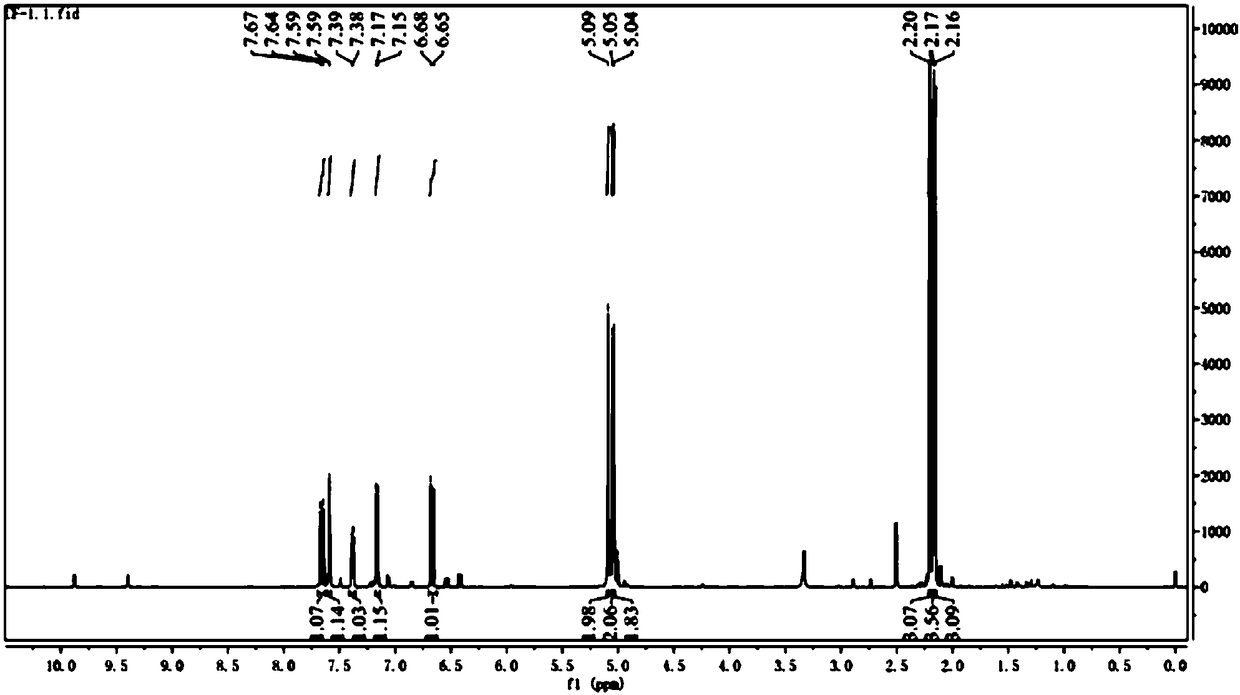
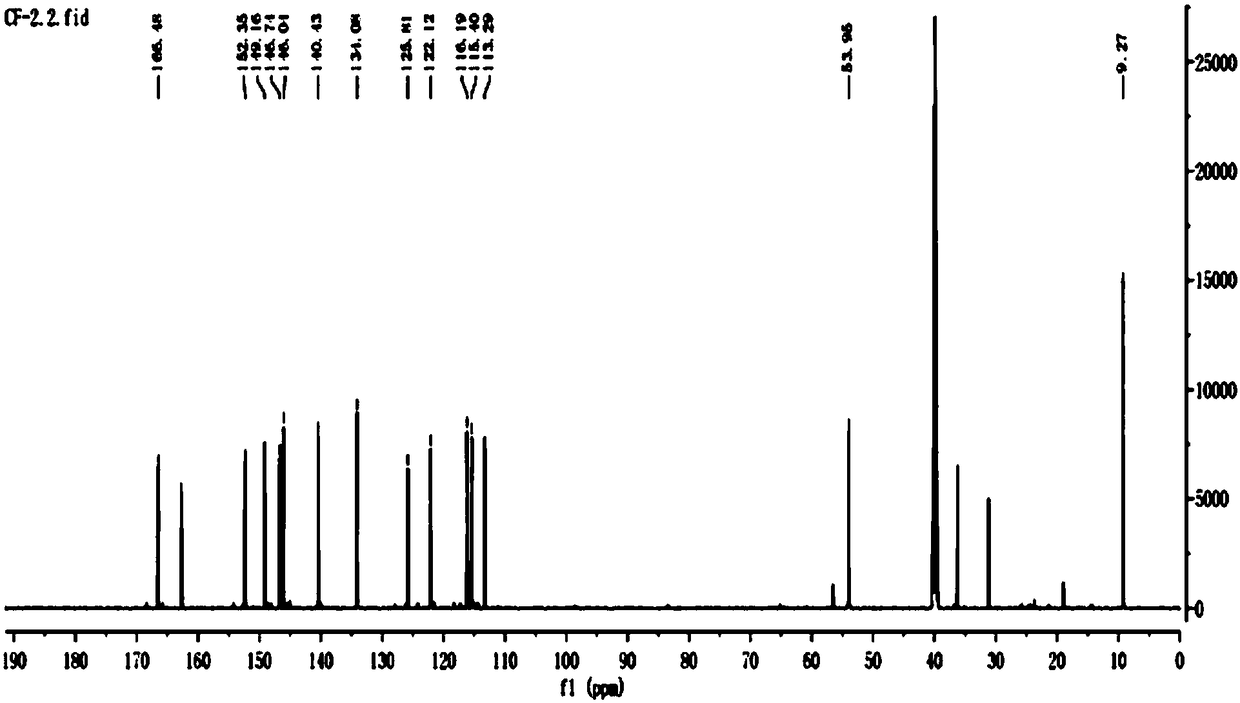
Image
Examples
Embodiment 1
[0052]
[0053] Reagent
[0054] Methanol analytically pure Nanjing Fuyu Fine Chemical Factory
[0055] Ethanol analytically pure Zhengzhou Damao Chemical Reagent Factory
[0056] Ethyl acetate Analytical grade Zhengzhou Damao Chemical Reagent Factory
[0057] Dichloromethane Analytical grade Tianjin Keyuan Industry & Trade Co., Ltd.
[0058] Chloroform Analytical Pure Nanjing Fuyu Fine Chemical Factory
[0059] Formic acid Analytical grade Tianjin Keyuan Industry & Trade Co., Ltd.
[0060] Dimethylformamide (DMF) Analytical grade Nanjing Fine Chemical Factory
[0061] Preparation of caffeic acid oleyl ester
[0062] Thin layer development conditions
[0063] The choice of developer: chloroform: methanol: formic acid = 9: 1: 0.5
[0064] The reaction chemical equation is as follows:
[0065]
[0066] 1. Take an analytical balance and weigh 0.0738g (0.0041mol) of caffeic acid raw material.
[0067] 2. Add the caffeic acid raw material into 3ml of anhydrous DMF, s...
Embodiment 2
[0081] Instrument, reagent are with embodiment 1.
[0082] Reaction formula:
[0083]
[0084] 1. Take an analytical balance and weigh 0.0738g (0.0041mol) of caffeic acid raw material. Add 3ml of anhydrous DMF, stir to make it completely dissolved.
[0085] 2. After stirring and adding 0.031 g (0.0022 mol) of potassium carbonate under ice bath conditions, stir in ice bath for two hours.
[0086] 3. Add 0.006g (0.0001mol) potassium iodide to the reaction solution under stirring in an ice bath, and then dropwise add 1 drop (about 0.033ml) 4-chloromethyl-5-methyl-2-oxo-1,3- After adding dioxole, the mixture was stirred in an oil bath.
[0087] 4. The mixture is gradually warmed up to 35°C within 1 hour, and stirred at 30-35°C for 2-3 hours. After the result of spotting the plate confirms that the reaction has been completed, the reaction is terminated.
[0088] 5. Slowly pour the above reaction solution into 10 ml of ice water with stirring.
[0089] 8. If there is no ob...
Embodiment 3
[0097] Three times of increasing dose experiments were carried out successively, and the mass of caffeic acid taken was: 0.738g; 7.38g; 21.56g (that is, magnified by ten times; one hundred times; three hundred times), and other reactions were added according to the ratio mentioned above. A total of about 29g of caffeic acid raw materials were put into the experiment.
[0098] product yield
[0099] CF-1:
[0100] Put in 29g of caffeic acid raw material, react through the above steps and then separate and purify by column chromatography, the quality of the obtained CF-1 is 10.2g, and the calculated yield is 35.2%.
[0101] CF-2:
[0102] Put in 29g of caffeic acid raw material, react through the above steps and separate and purify by column chromatography, the quality of the obtained CF-2 is 16.6g, and the calculated yield is 57.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com