Non-aqueous reversed-phase chromatographic detection method of borate compounds
A technology of borate esters and reversed-phase chromatography, which is applied in the field of liquid chromatography non-aqueous reversed-phase detection of borate compounds. Good shape, fast separation speed and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
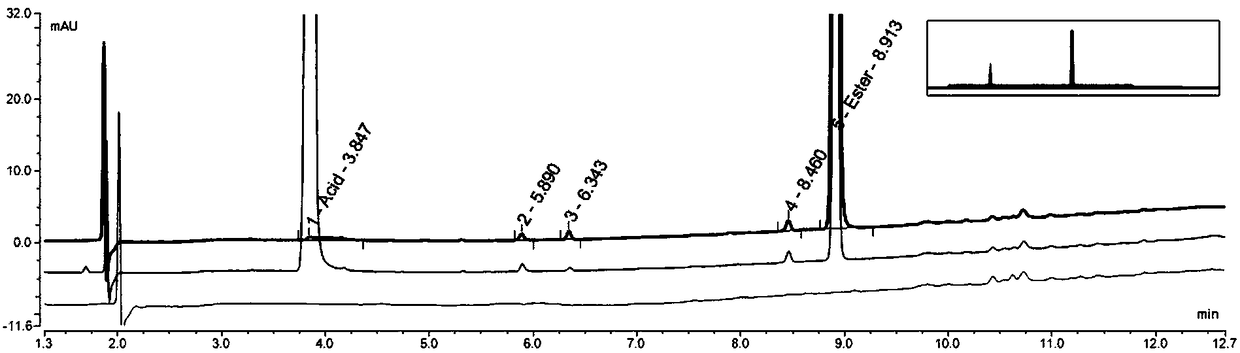
[0166] Example 1: The laboratory uses conventional reversed-phase liquid chromatography instrument Thermo U-3000, chromatographic grade solvent acetonitrile, tetrahydrofuran, etc., and uses Thermo Hypercarb (150mm*4.6mm, 5.0um) column for separation and analysis:
[0167] step one
[0168] Tetrahydrofuran acetonitrile is used as mobile phase, and acetonitrile is used as solvent to dissolve samples to detect stability;
[0169] step two
[0170] Reagents: tetrahydrofuran, acetonitrile (chromatographically pure), ultrapure water
[0171] Instrument: Thermo U-3000
[0172] Chromatographic column: Thermo Hypercarb (150mm*4.6mm, 5.0um)
[0173] Column temperature: 35°C
[0174] mobile phase:
[0175] Phase A: tetrahydrofuran
[0176] Phase B: Acetonitrile
[0177] Gradient program:
[0178] Time(min) A% B%
[0179] 0.00 10 90
[0180] 10.00 10 90
[0181] Flow rate: 1.0ml / min
[0182] Detector wavelength: UV 220nm
[0183] Injection volume: 5μl
[0184] Needle washing...
example 2
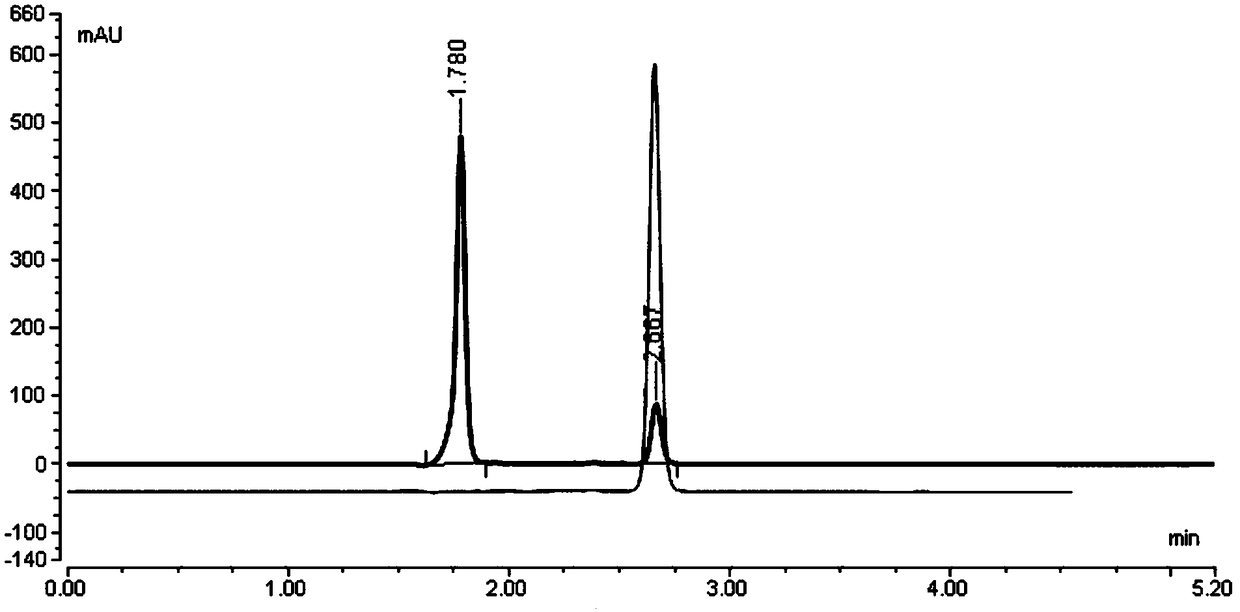
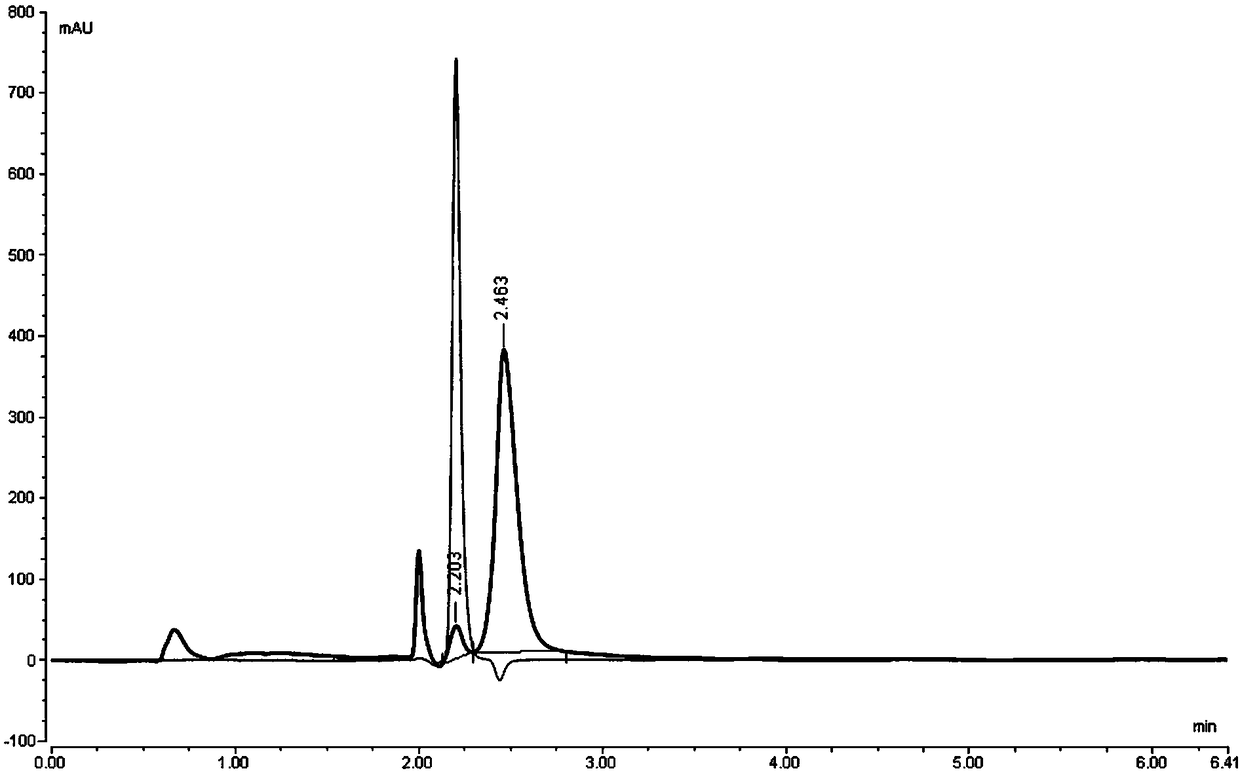
[0194] Example 2: The laboratory uses conventional reversed-phase liquid chromatography instrument Thermo U-3000, chromatographic grade solvent acetonitrile, tetrahydrofuran, etc., and uses Thermo Hypercarb (150mm*4.6mm, 5.0um) column for separation and analysis to optimize the compound of formula II.
[0195] step one
[0196] Dichloromethane and acetonitrile were used as mobile phase, and acetonitrile was used as solvent to dissolve the sample, and the stability was tested.
[0197] step two
[0198] Reagents: dichloromethane, acetonitrile (chromatographically pure), ultrapure water
[0199] Instrument: Thermo U-3000
[0200] Chromatographic column: Thermo Hypercarb (150mm*4.6mm, 5.0um)
[0201] Column temperature: 25°C
[0202] mobile phase:
[0203] Phase A: dichloromethane
[0204] Phase B: Acetonitrile
[0205] Gradient program:
[0206] Time(min) A% B%
[0207] 0.00 20 80
[0208] 10.00 20 80
[0209] Flow rate: 0.5ml / min
[0210] Detector wavelength: UV 230...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com