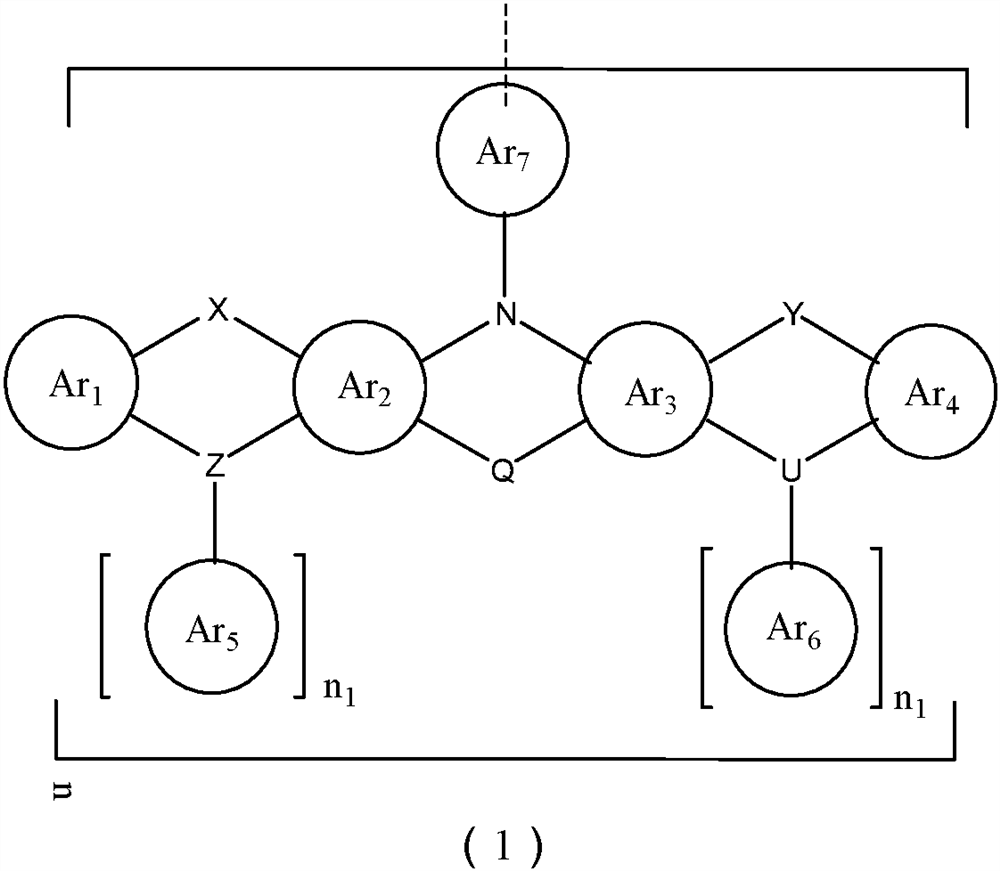
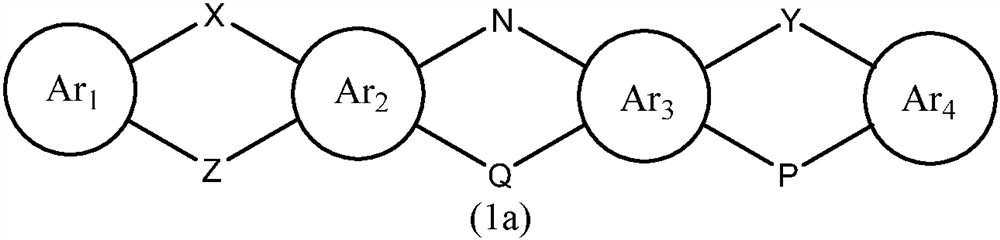
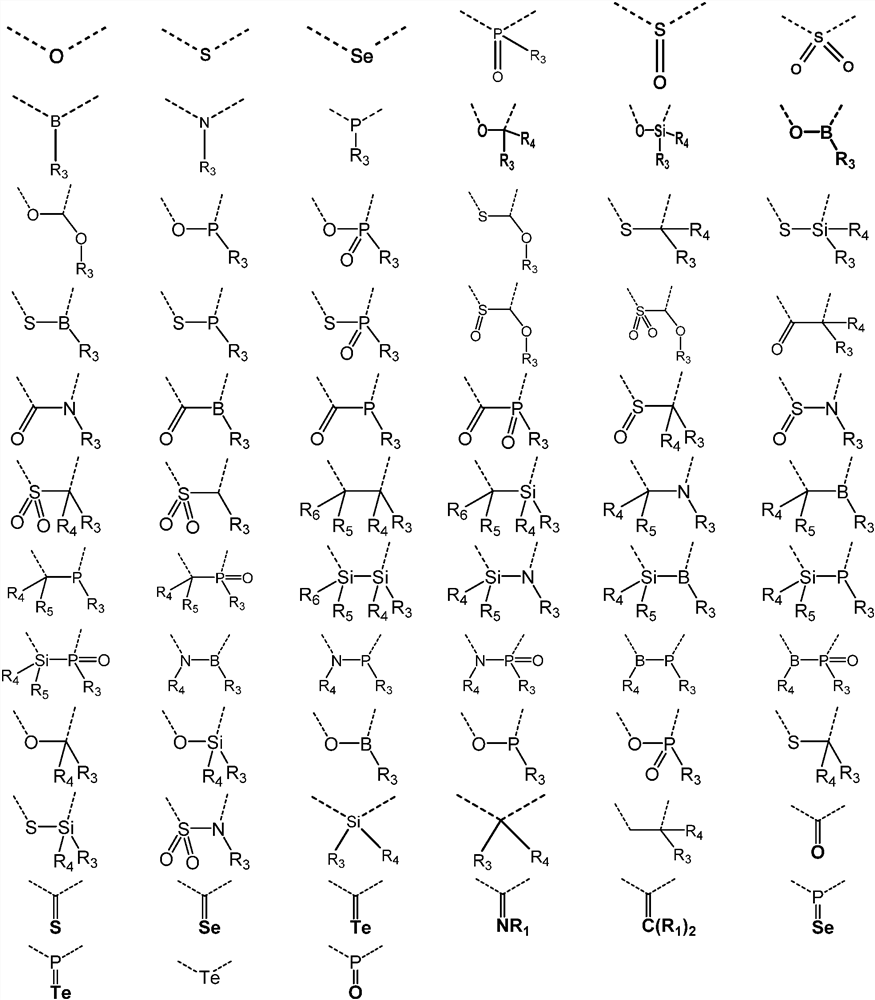
A kind of organic photoelectric material and its application
An organic electronic device, organic technology, applied in electroluminescent devices, the application in organic electroluminescent devices, the field of organic electronic devices, can solve the problems of fast efficiency roll-off, short life, low fluorescence quantum yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190]
[0191]
[0192] 15-(4,6-diphenyl-1,3,5-triazinyl-2-yl)-5,10-diphenyl-10,15-dihydro-5H-pyrrole[3,2-c : 4,5-c'] dicarbazole
[0193] Add 4.98g, 10mmol 5,10-diphenyl-10,15-dihydro-5H-pyrrole[3,2-c:4,5-c']dicarbazole, 0.36g, 15mmol hydrogenation to a 250ml three-necked flask Sodium, 100ml THF, in N 2 In the atmosphere, stir at room temperature for 30min, add 2.97g, 11mmol of 2-chloro-4,6-diphenyl-1,3,5-triazine in batches, and react at 60°C for 12 hours. Bring to room temperature. The reaction solution was poured into water, washed to remove sodium hydride, and then suction filtered to obtain a solid product, which was dissolved in dichloromethane to remove impurities therein. Recrystallize with toluene / ethanol to obtain the product solid powder 15-(4,6-diphenyl-1,3,5-triazinyl-2-yl)-5,10-diphenyl-10,15-di Hydrogen-5H-pyrrole[3,2-c:4,5-c']biscarbazole 6.2 g. MS (ASAP) = 729.3.
Embodiment 2
[0195]
[0196] 5,10-Diphenyl-15-(4′-(11-phenylindole[3,2-b]carbazole-5(11H)-yl)-[1,1′-diphenyl] -4-yl)-10,15-dihydro-5H-pyrrole[3,2-c:4,5-c']bicarbazole
[0197] Add 4.98g, 10mmol 5,10-diphenyl-10,15-dihydro-5H-pyrrole [3,2-c:4,5-c']dicarbazole, 6.2g, 11mmol 5- (4'-bromo-[1,1'-diphenyl]-4-yl)-11-phenyl-5,11-dihydroindolo[3,2-b]carbazole, 6.9g, 50mmol carbonic acid Potassium, 0.26g, 1mmol 18-crown-6, 3.0g, 15mmol cuprous iodide and 150ml o-dichlorobenzene, in N 2 In the atmosphere, react at 160°C, track the progress of the reaction by TLC, and cool down to room temperature after the reaction is completed. Pour the reaction solution into water, wash to remove K 2 CO 3 , and then suction filtered to obtain a solid product, which was washed with dichloromethane. Recrystallize with dichloromethane and ethanol to obtain the product 5,10-diphenyl-15-(4'-(11-phenylindole[3,2-b]carbazole-5(11H)-yl) -[1,1'-diphenyl]-4-yl)-10,15-dihydro-5H-pyrrole[3,2-c:4,5-c']bicarbazole 8.0g,...
Embodiment 3
[0199]
[0200] 15-(3-(4-([1,1′:3′,1″-tetraphenyl]-5′-yl)-6-phenyl-1,3,5-triazinyl-2-yl )phenyl)-5,10-diphenyl-10,15-dihydro-5H-pyrrole[3,2-c:4,5-c']biscarbazole
[0201] Add 4.98g, 10mmol5,10-diphenyl-10,15-dihydro-5H-pyrrole[3,2-c:4,5-c']dicarbazole, 5.94g, 11.0mmol2 to a 250ml three-necked flask -([1,1′:3′,1″-tetraphenyl]-5′-yl)-4-(3-bromophenyl)-6-phenyl-1,3,5-triazine, 6.9 g, 50mmol potassium carbonate, 0.26g, 1mmol18-crown-6, 3.0g, 15mmol cuprous iodide and 150ml o-dichlorobenzene, in N 2 In the atmosphere, react at 160°C, track the progress of the reaction by TLC, and cool down to room temperature after the reaction is completed. Pour the reaction solution into water, wash to remove K 2 CO 3, and then suction filtered to obtain a solid product, which was washed with dichloromethane. Recrystallize with a mixed solvent of toluene / petroleum ether to obtain the product white solid powder 15-(3-(4-([1,1′:3′,1″-tetraphenyl]-5′-yl)-6-benzene Base-1,3,5-triazinyl-2-yl)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com