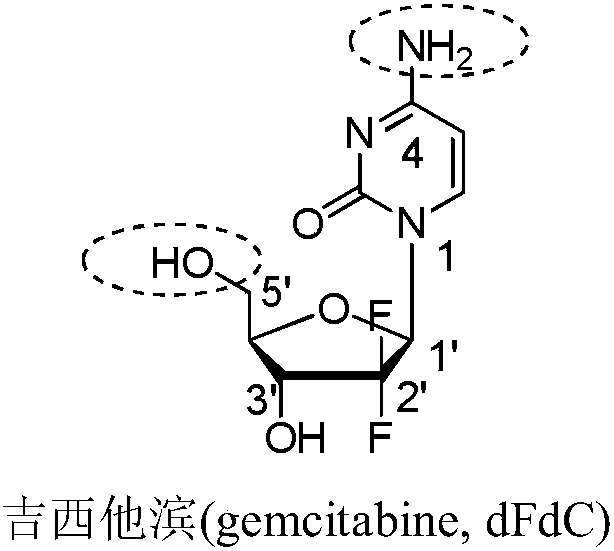
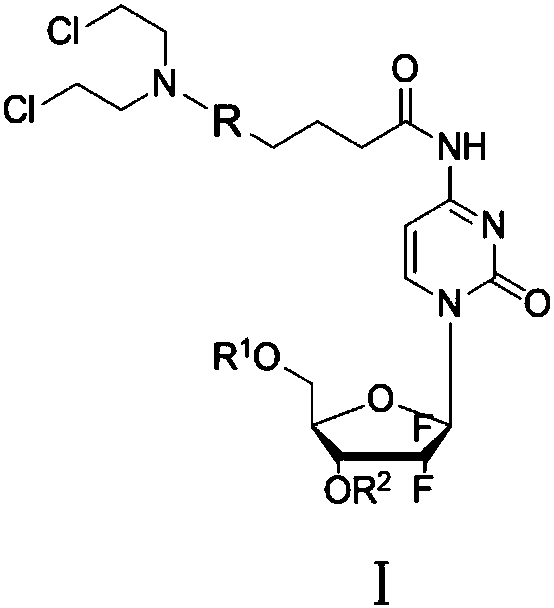
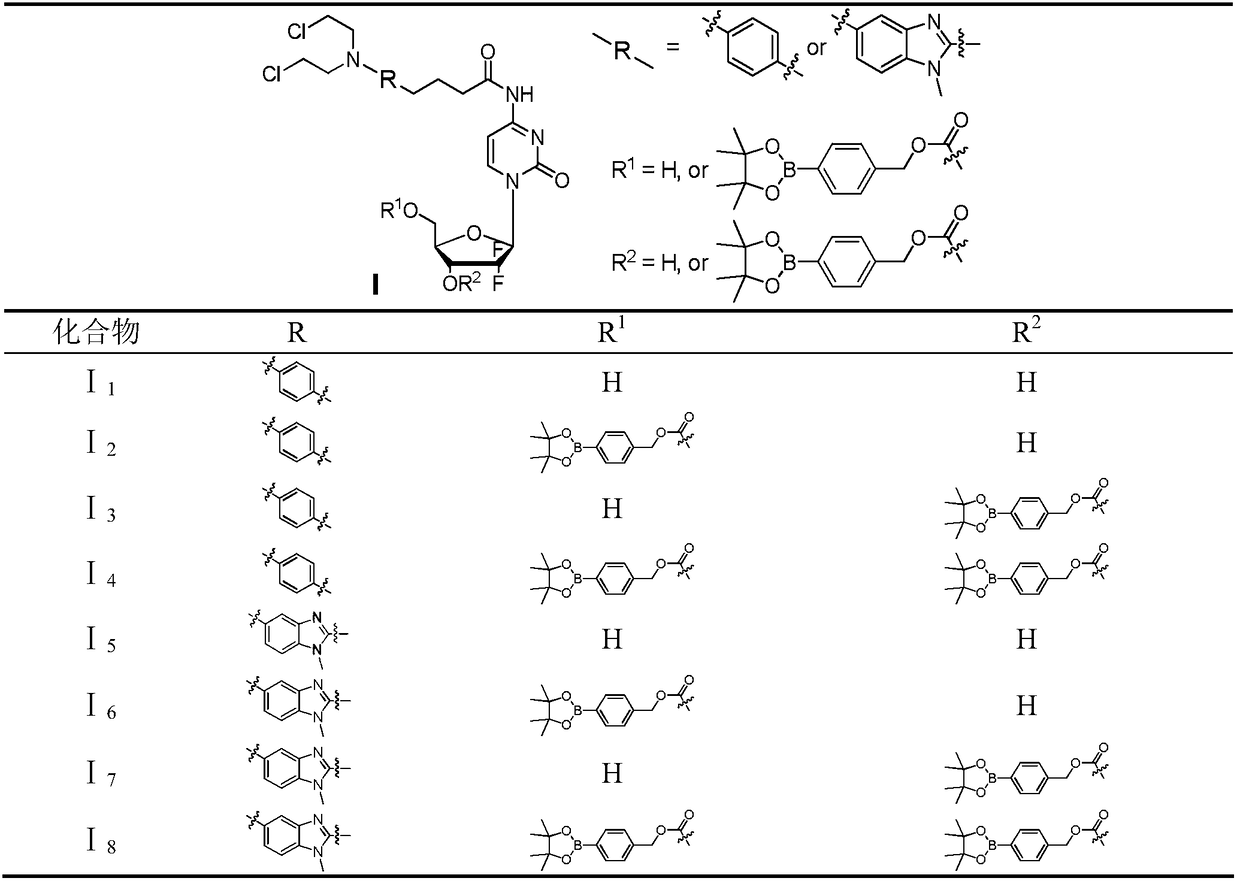
Gemcitabine-aromatic chlormethine conjugate targeting at high-level ROS (reactive oxygen species) of cancer cells and preparation method and pharmaceutical application thereof
A cancer cell, high-level technology, applied in the field of biomedicine, can solve the problems of high toxicity, lack of selectivity, and low bioavailability in normal tissues, improve lipid solubility and transmembrane ability, reduce drug resistance, and improve biological The effect of utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 4-(4-(bis(2-chloroethyl)amino)phenyl)-N-(1-((2R,4R,5R)-3,3-difluoro-4-hydroxyl-5- (Hydroxymethyl)tetrahydrofuran-2-yl)-2-one-1,2-dihydropyrimidin-4-yl)-butanamide (I 1 ) preparation
[0051] 4-amino-1-((2R,4R,5R)-3,3-difluoro-4-(tert-butyldimethylsilyloxy)-5-(tert-butyldimethylsilyloxymethyl ) Tetrahydrofuran-2-yl)-1H-pyrimidin-2-one (compound 4) preparation
[0052] Gemcitabine (compound 3, 1.0g, 3.80mmol) was dissolved in 10mL dry DMF, TBDMS-Cl (1.15g, 7.64mmol) and 1.03g imidazole were added, stirred at room temperature, after the reaction was detected by thin layer chromatography, added 100ml of water, and an equal amount of ethyl acetate to extract three times, the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a crude product. The crude product was purified by column chromatography (mobile phase methanol:dichloromethane=1:25-1:15) to obtain 1.64g, yield 88%.
[0053]
[0054] 4-(4-(bis(2-ch...
Embodiment 2
[0058] Example 2 ((2R,3R,5R)-5-(4-(4-(4-(bis(2-chloroethyl)amino)phenyl)butanylamino-2-oxopyrimidine-1(2H )-yl-4,4-difluoro-3-hydroxytetrahydrofuran-2-yl)methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane- 2-yl)benzyl carbonate (I 2 ), (2R,3R,5R)-5-(4-(4-(4-(bis(2-chloroethyl)amino)phenyl)butanylamino-2-oxopyrimidine-1(2H)- Base)-4,4-difluoro-2-(hydroxymethyl)tetrahydrofuran-3-yl)methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaboron Pentyl-2-yl)benzyl carbonate (I 3 ) and ((2R,3R,5R)-5-(4-(4-(4-(bis(2-chloroethyl)amino)phenyl)butanylamino-2-oxopyrimidine-1(2H) -yl)-4,4-difluoro-3-((((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl )oxy)carboxy)oxy)tetrahydrofuran-2-yl)methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl base) carbonate (I 4 ) preparation
[0059] Preparation of (4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methanol (compound 2)
[0060] Dissolve p-bromobenzyl alcohol (compound 1, 1.0 g, 5.35 mmol) in 10 mL of dry 1,4-dioxane solution, ad...
Embodiment 3
[0067] Example 3 4-(5-(bis(2-chloroethyl)amino)-1-methyl-1H-benzo[d]imidazol-2-yl)-N-(1-((2R,4R, 5R)-3,3-difluoro-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-2-one-1,2-dihydropyrimidin-4-yl)-butyramide (I 5 ) preparation
[0068] 4-(5-(bis(2-chloroethyl)amino)-1-methyl-1H-benzo[d]imidazol-2-yl)-N-(1-((2R,4R,5R)- 4-((tert-butyldimethylsilyl)oxy)-5-(((tert-butyldimethylsilyl)oxy)methyl)-3,3-difluorotetrahydrofuran-2-yl) Preparation of -2-keto-1,2-dihydropyrimidin-4-yl)-butyramide (compound 6b)
[0069] Bendamustine hydrochloride (compound 5b) (454 mg, 1.10 mmol), EDCI (384, 2.00 mmol), DMAP (227 mg, 50%) and compound 4 (492 mg, 1.00 mmol) were dissolved in 10 ml of anhydrous CH 2 Cl 2 , heated at 40°C for 12 hours. After the reaction, the solvent was distilled off under reduced pressure, and the crude product was purified by column chromatography (mobile phase ethyl acetate:petroleum ether=2:1-4:1) to obtain 656mg of a light yellow solid with a yield of 79%. %.
[007...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com