Triketone-based compound preparation method
A technology for compounds and triketones, which is applied in the field of preparing triketone compounds, can solve the problems of hindered synthesis of homogentisic acid, complicated process routes and high commercial cost of compounds, and achieves the effects of low cost and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
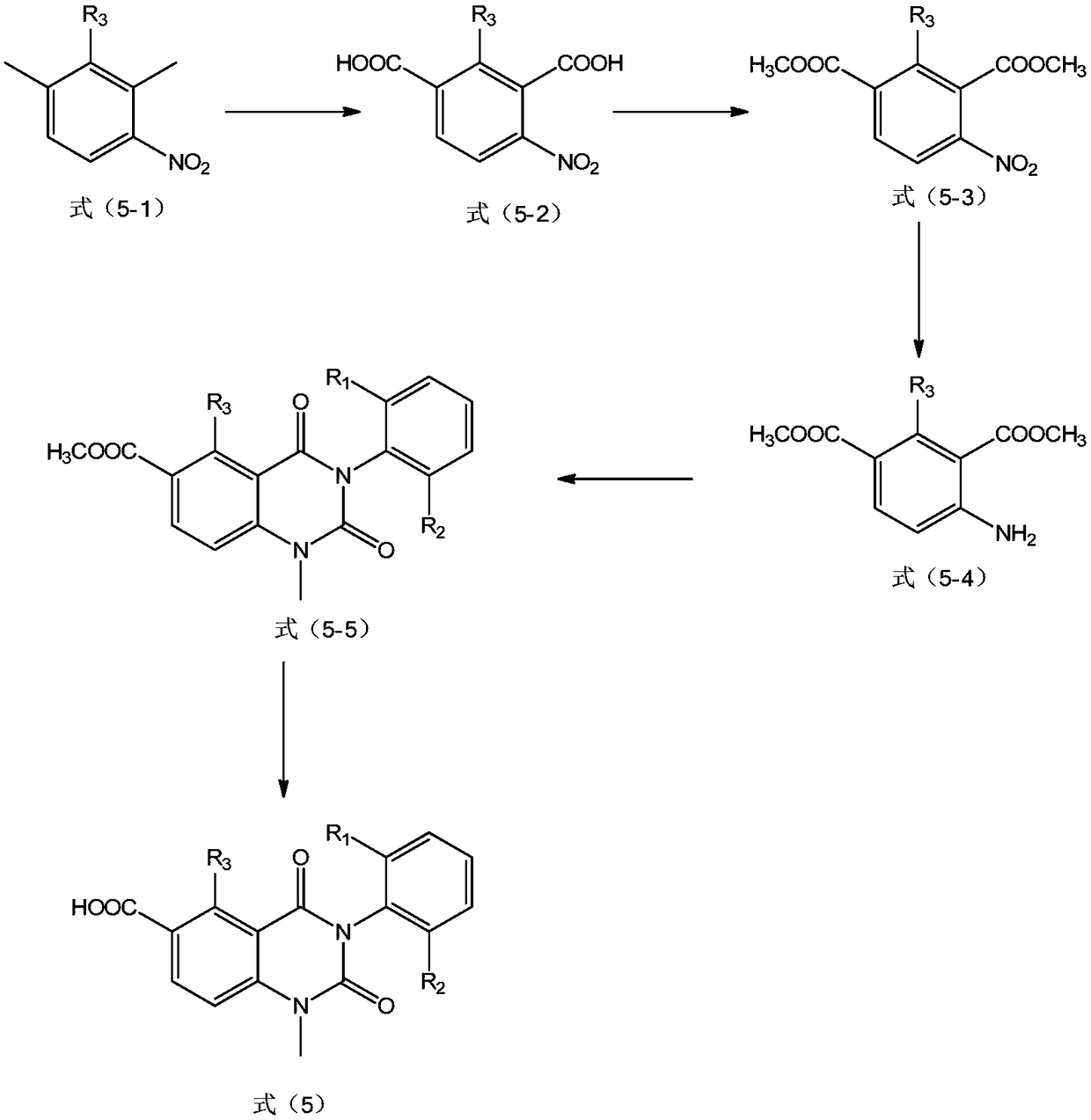
[0094] Preparation example 1: using figure 1 The compound shown in the route synthetic formula (5) shown, wherein, R 3 for H, R 1 and R 2 for methyl
[0095] 1. Add water (500mL) into a 1000mL four-necked flask, start stirring, cool the system to 5°C with ice-salt water, then slowly add potassium hydroxide (553mmol) into a 100mL four-necked flask, and the addition is complete Finally, remove the icy brine, and slowly add 2,4-dimethylnitrobenzene (3311 mmol) dropwise. After the dropwise addition, the reaction system is heated to 90°C. After the reaction system rose to 90°C, potassium permanganate (1659mmol) was added to the four-necked flask in batches within 0.5h. After the addition was complete, the reaction system was kept at 90°C for 3h, and the reaction was followed by HPLC. After the reaction is completed, filter while hot and wash the filter cake with 500 mL of hot water (70° C.). The filtrate was cooled to 5° C., and concentrated hydrochloric acid (36% by weight, 1...
preparation example 2
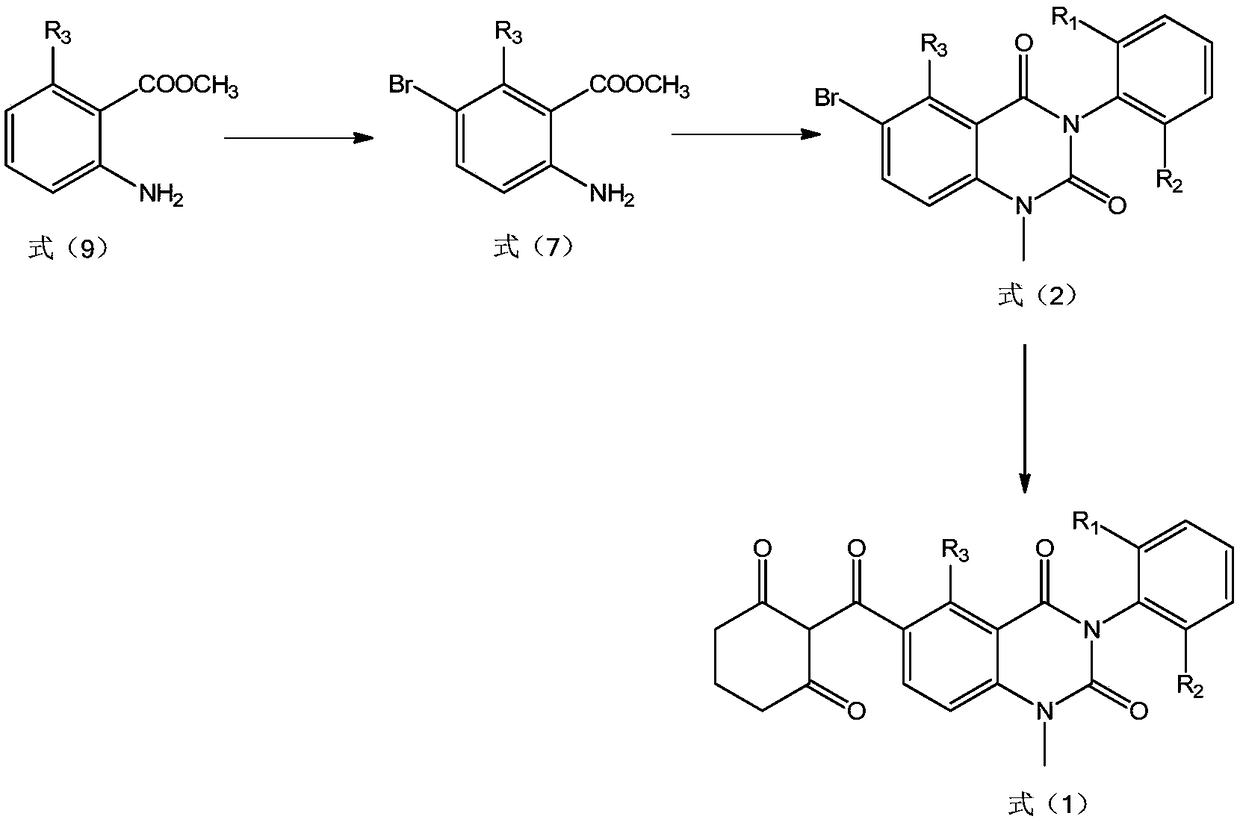
[0100] Preparation example 2: the compound shown in preparation formula (2), and R 1 and R 2 is methyl, R 3 for H
[0101] 1. Add carbon tetrachloride (100mL) and methyl 2-aminobenzoate (149mmol) into a 250mL four-necked flask, start stirring, and start to add bromine (156.45mmol) dropwise. After the dropwise addition, take a sample by HPLC Track reactions. After the reaction was completed, the reaction solution was filtered, the filter cake was rinsed with 200 mL of isopropyl ether, and dried to obtain a yellow solid with a purity of 96.5% and a yield of 90%.
[0102] 2. Add pyridine (250mL) and the product obtained in step 1 (144mmol) into a 500mL four-neck flask, start stirring, then add 2,6-dimethylphenylisocyanate (179mmol), after the addition is complete, the reaction system Raise the temperature to 100°C and keep it overnight, and track the reaction by HPLC. After the reaction was completed, the reaction solution was poured into 1 L of water while it was hot, stirr...
preparation example 3
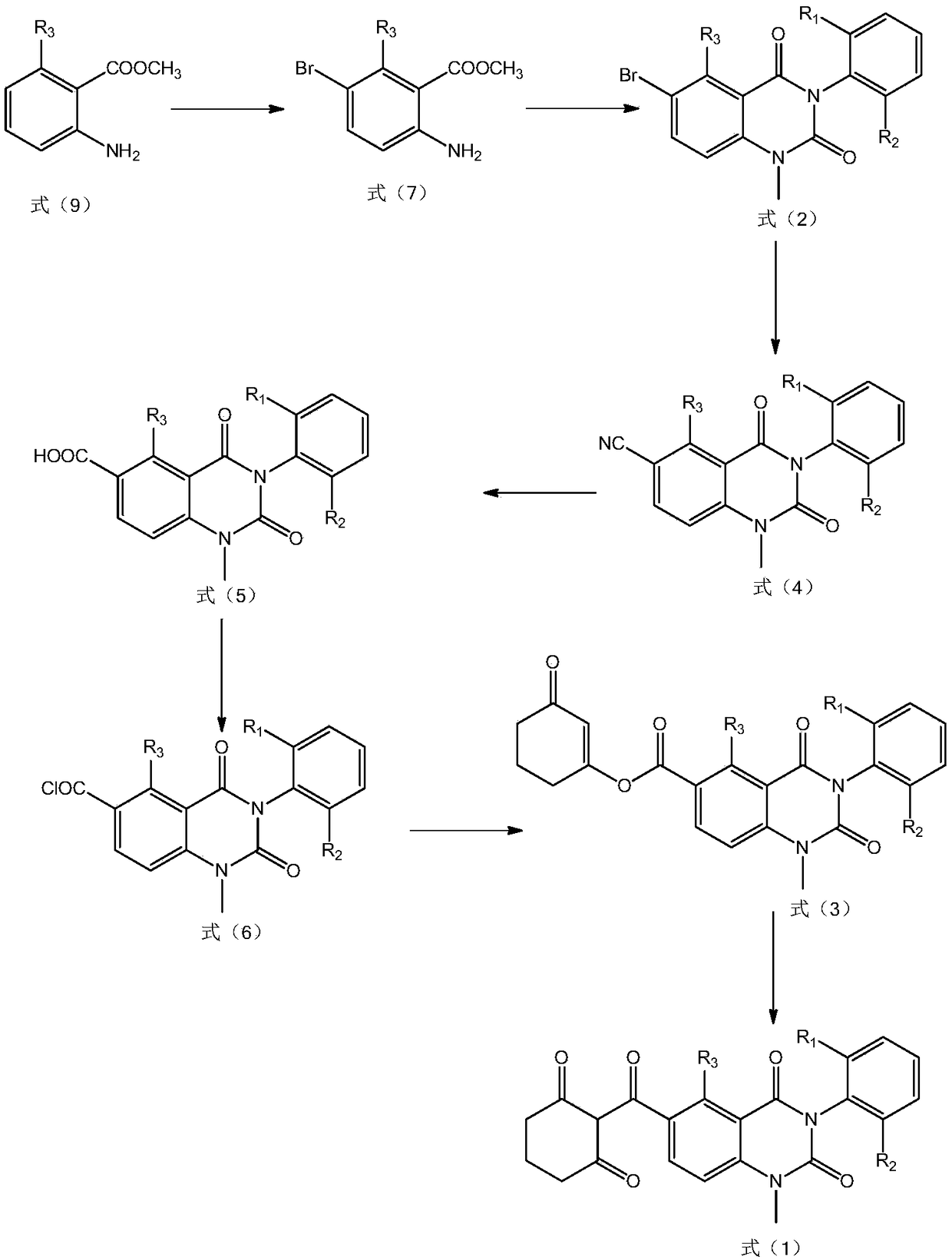
[0103] Preparation example 3: the compound shown in preparation formula (4), and R 1 and R 2 is methyl, R 3 for H
[0104] Add DMF (300mL) and methyl 2-amino-5-bromobenzoate (130.4mmol) into a 1000mL single-necked bottle, start stirring, and then add cuprous cyanide (2260.8mmol). After the addition was complete, the reaction system was heated to reflux and kept overnight, and the reaction was tracked by HPLC. After the reaction is completed, lower the reaction solution to 25°C, then add 500 mL of water to the reaction solution and stir for 30 minutes, then add 300 mL of ethyl acetate to it, filter after the stirring is completed, and let the filtrate stand for stratification. The aqueous phase was extracted three times with 600 mL of ethyl acetate, and the organic phase was dried with anhydrous sodium sulfate and precipitated to obtain a white solid with a purity of 95.6% and a yield of 90%.
[0105] 2. Add pyridine (167mL), the product obtained in step 1 (113.6mmol) into ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com