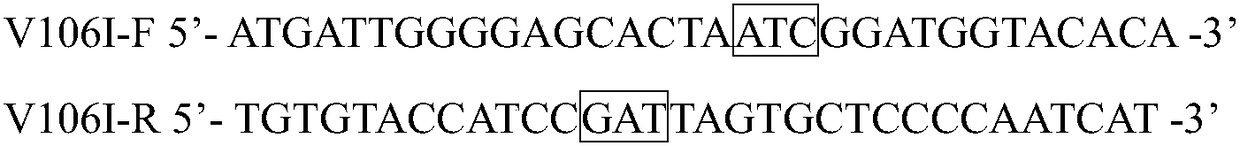Kidney bean epoxide hydrolase mutant with improved catalytic activity
A technology of epoxides and mutants, applied in the fields of genetic engineering and protein expression, can solve problems such as low enzyme activity and limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Construction of mutant enzyme gene and its expression plasmid
[0021] 1. Acquisition of plasmid pET-28a(+)-pveh1
[0022] The medium composition of recombinant E.coli BL21(DE3) / pET-28a(+)-pveh1 is: peptone 1%, yeast extract 0.5%, NaCl 1%.
[0023] The recombinant bacteria were inoculated in a test tube with a medium filling volume of 5 mL and cultured at 37° C. and 215 rpm for 12 h with shaking. After the cultivation, the cells were centrifuged at 12,000 rpm for 1 min and the cells were collected, and the plasmid pET-28a(+)-pveh1 was extracted using the plasmid extraction kit Pure PlasmidMini Kit (Kangwei Century Biotechnology Co., Ltd.); the plasmid pET-28a The amino acid sequence of the epoxide hydrolase PvEH1 in (+)-pveh1 is shown in SEQ ID NO.2 (the nucleotide sequence is shown in SEQ ID NO.4).
[0024] 2. Construction of recombinant Escherichia coli E. coli BL21(DE3) / pET-28a(+)-pveh1(V106I)
[0025] Design and synthesize specific site-directed mut...
Embodiment 2
[0029] Example 2: Mutant enzyme PvEH1 V106I the acquisition of
[0030] E.coli BL21-pveh1 V106I Inoculate a single colony in 2 mL of LB medium containing 100 μg / mL kanamycin, and culture overnight at 37°C and 215 r / min; transfer 1 mL of the culture solution to 50 mL of the same medium, and culture to OD 600 When it is 0.6-0.8, add IPTG (final concentration 0.5mmol / L) and induce at 20°C for 10h. Collect the thalli, use 10mL sodium phosphate buffer (Na 2 HPO 4 -NaH 2 PO 4 , 100mmol / L, pH 7.0) to obtain a bacterial suspension.
Embodiment 3
[0031] Example 3: Mutant enzyme PvEH1 V106I Determination of catalytic activity
[0032] Add 30 μL of bacterial suspension and 920 μL of phosphate buffer to a 2 mL EP tube, incubate at 25°C for 5 min, then add 50 μL of racemic o-methylphenyl glycidyl ether (rac-o-GMPE, final concentration 10 mmol / L) immediately After timing the reaction for 10 min, take 200 μL of the reaction solution and add 1 mL of ethyl acetate to terminate the reaction. After microfiltration, samples were analyzed by normal phase HPLC, OD-H column and UV detector. The mobile phase was n-hexane / isopropanol (80:20, v / v), the flow rate was 0.8 mL / min, and the detection wavelength was 220 nm. Definition of enzyme activity unit: Under the conditions of this assay, the amount of enzyme required to decompose 1 μmol rac-o-GMPE per minute is defined as an activity unit (IU) of epoxide hydrolase. Mutant enzyme PvEH1 V106I The catalytic activity was 234.75U / g, which was 1.49 times that of the wild-type enzyme (15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com