Allylestrenol tablet and preparation method thereof
A technology of allylestradiol tablets and allylestradiol, which is applied in the directions of pill delivery, pharmaceutical formulations, medical preparations of inactive ingredients, etc., can solve the problems of small size, difficult mixing and the like, and achieves low cost and increased Efficacy of efficacy, improved solubility and dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
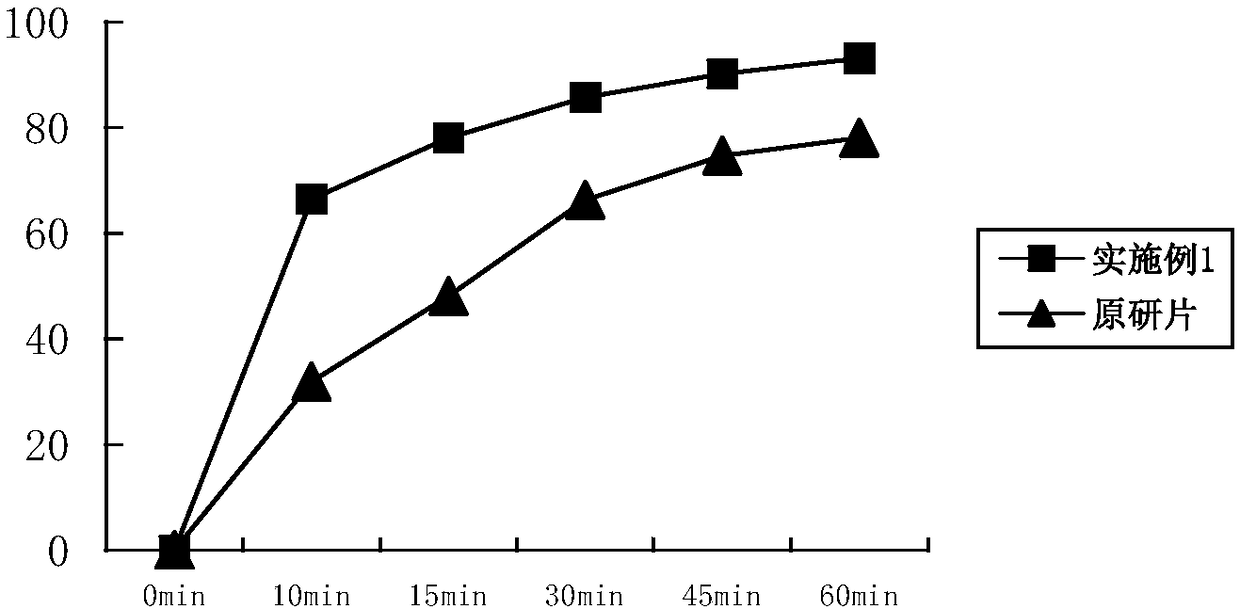
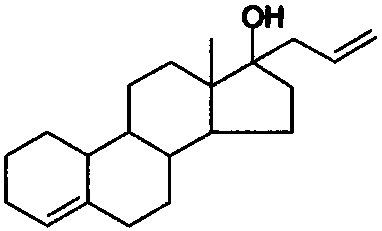
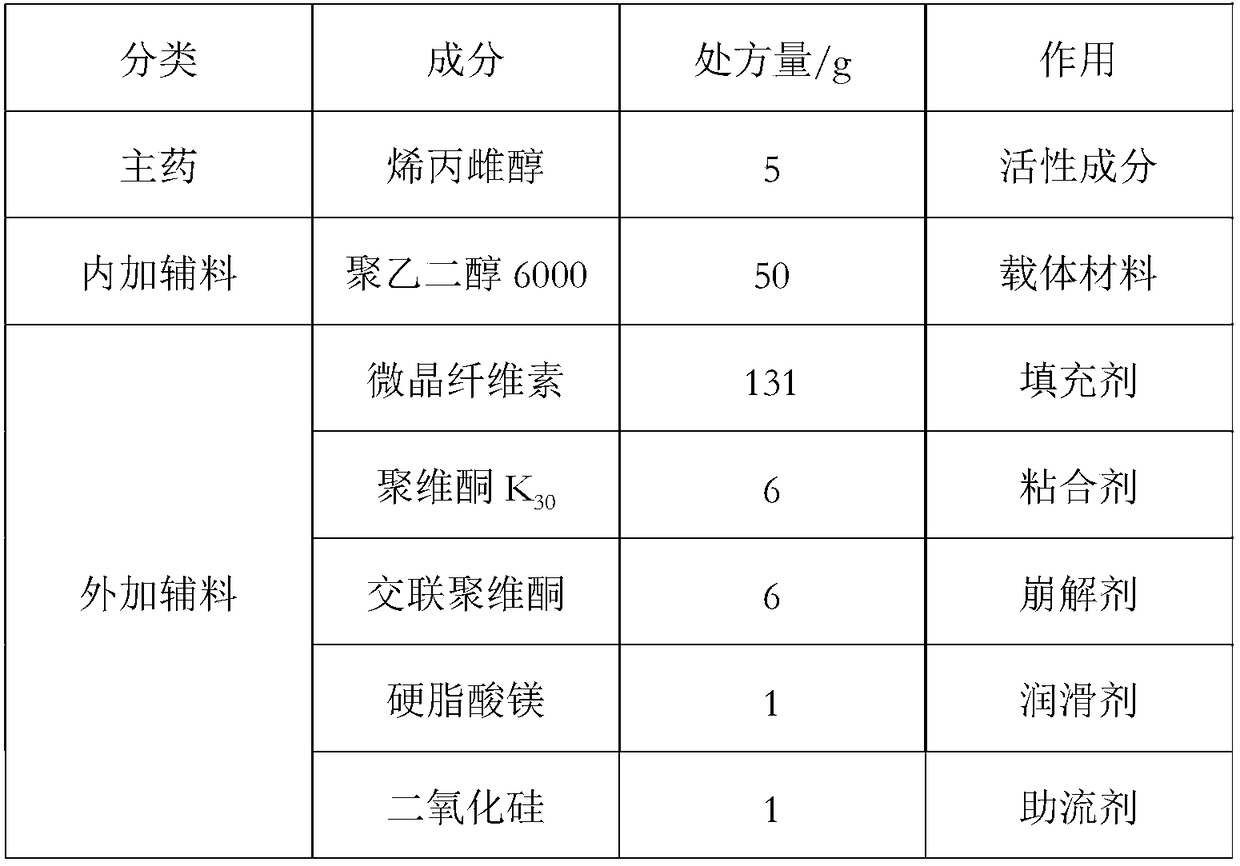
[0030] Allylestradiol tablets (5 mg / tablet) were prepared using the ingredients listed in Table 1, 1000 tablets.
[0031] Table 1:
[0032]
[0033] Preparation Process:
[0034] Mix the allylestradiol raw material with polyethylene glycol 6000, put it into a hot melt extruder, control the heating temperature at 100-120°C, adjust the discharge speed so that the residence time of the material in the heating part of the equipment is 30-30°C 50 seconds. Cut the extrudate into small pieces and cool. After crushing with a pulverizer, add crospovidone and povidone K 30 , microcrystalline cellulose and mix evenly, then add silicon dioxide and magnesium stearate and mix for 5 minutes, press into tablets, and obtain.
Embodiment 2
[0036] Allylestradiol tablets (5 mg / tablet) were prepared using the ingredients listed in Table 2, 1000 tablets.
[0037] Table 2:
[0038]
[0039]
[0040] Preparation Process:
[0041] Mix the allylestradiol raw material with polyethylene glycol 4000, put it into a hot melt extruder, control the heating temperature at 90-130°C, adjust the discharge speed so that the residence time of the material in the heating part of the equipment is 20-20°C 60 seconds. Cut the extrudate into small pieces and cool. After crushing with a pulverizer, add sodium carboxymethyl starch and povidone K 30 , microcrystalline cellulose and mix evenly, then add silicon dioxide and magnesium stearate and mix for 5 minutes, press into tablets, and obtain.
Embodiment 3
[0043] Allylestradiol tablets (5 mg / tablet) were prepared using the ingredients listed in Table 3, 1000 tablets.
[0044] table 3:
[0045]
[0046] Preparation Process:
[0047] Mix the allylestradiol raw material with polyethylene glycol 6000, put it into a hot melt extruder, control the heating temperature at 90-130°C, adjust the discharge speed so that the residence time of the material in the heating part of the equipment is 20-20°C 60 seconds. Cut the extrudate into small pieces and cool. After pulverizing with a pulverizer, add crospovidone, hydroxypropyl methylcellulose, and lactose to mix evenly, then add silicon dioxide and magnesium stearate, mix for 5 minutes, and press into tablets to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com