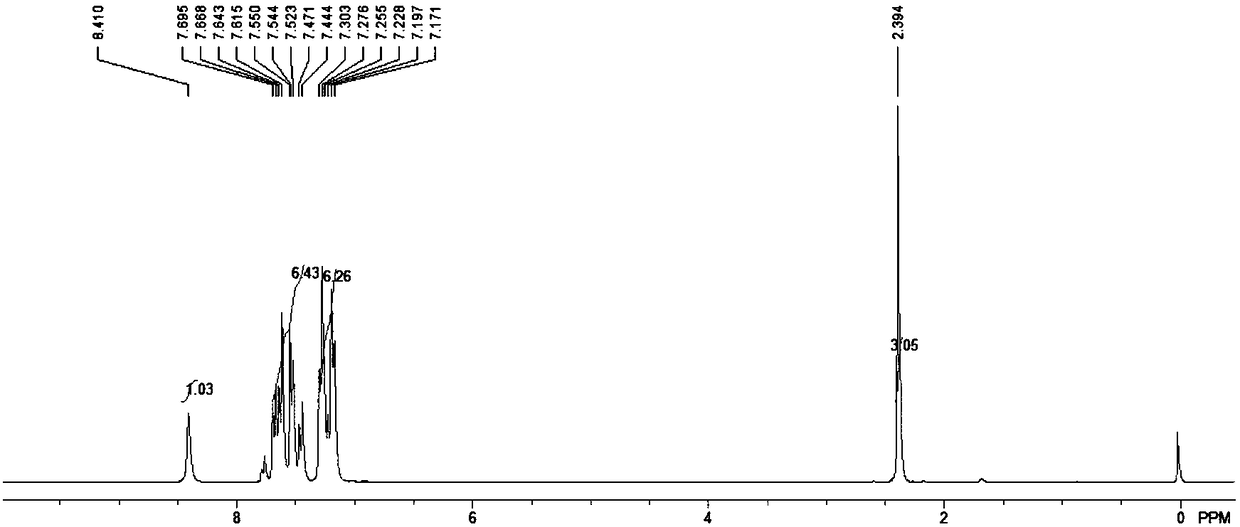
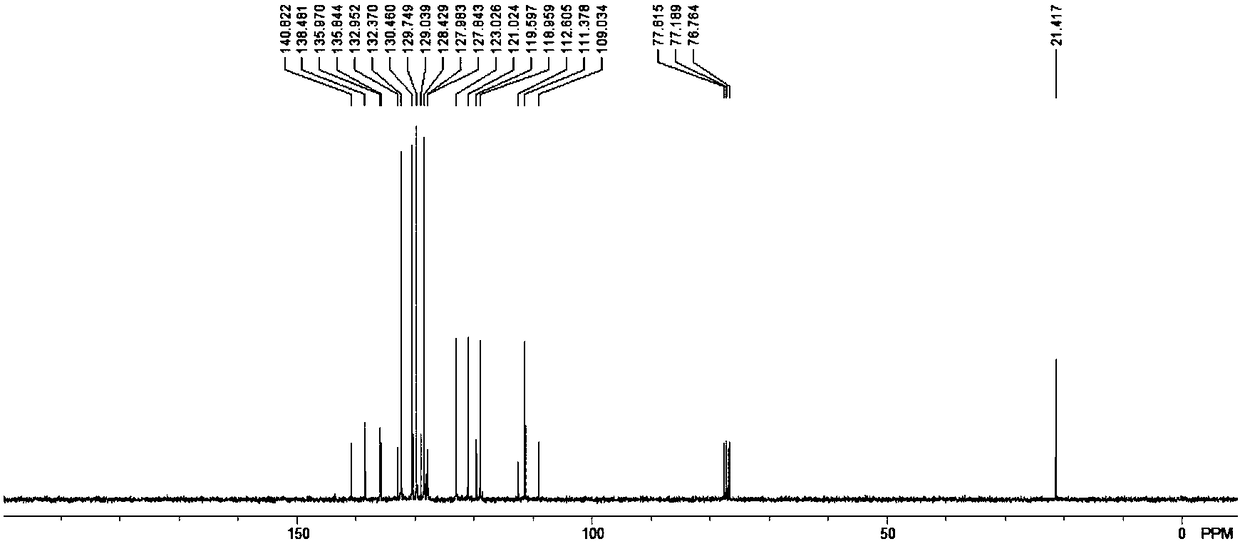
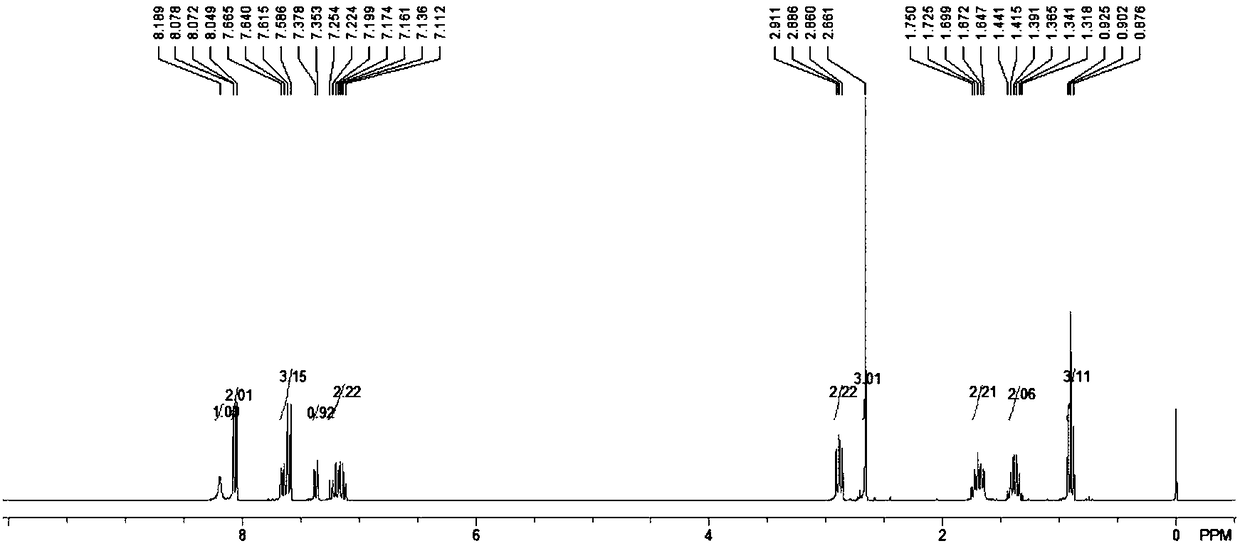
2,3-disubstituted indole derivative and preparation method thereof
An indole and disubstituted technology, applied in the field of 2,3-disubstituted indole derivatives and their preparation, can solve the problems of expensive raw material sources, low reaction yield, long reaction steps, etc., and achieve wide application The effect of prospect, simple preparation method and high compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention proposes a method for preparing 2,3-disubstituted indole derivatives, which includes: constructing 2,3-disubstituted indole derivatives through palladium-catalyzed cascade reactions with alkynyl anilines and substituted bromobenzenes of different structures compound.
[0028] Further, in a preferred embodiment of the present invention, a method for preparing a 2,3-disubstituted indole derivative provided by the present invention comprises the following steps:
[0029] a. Precursor compound synthesis;
[0030] b. Synthesis of the target product;
[0031] c. Purification.
[0032] Wherein, step a, b synthetic route is as follows:
[0033]
[0034] Step a comprises the following steps: in an anhydrous and oxygen-free system, adding o-alkynyl aniline and trifluoroacetic acid into a carbon tetrachloride solvent, adding triphenylphosphine as a catalyst, and adding a certain amount of triethylamine, ice Stir in a water bath environment for 10 minut...
Embodiment 1
[0046] A preparation method of 2,3-disubstituted indole derivatives comprises the following steps:
[0047] a. Precursor compound synthesis:
[0048] In an anhydrous and oxygen-free system, add 20mmol o-tolylethynylaniline, 20mmol trifluoroacetic acid and 40ml carbon tetrachloride solvent into a 100ml reaction flask, then add 50mmol triphenylphosphine and 22mmol triethylamine, and place in an ice-water bath Stir in the environment for 10 minutes, then heat and reflux for 3 hours, stop the reaction, add water to wash several times, then extract and concentrate with ethyl acetate, dry with anhydrous magnesium sulfate, and finally use acetic acid with a volume ratio of 1: (40-80) The mixture of ethyl acetate and petroleum ether was used as the eluent for column chromatography separation and purification. The volume ratio of ethyl acetate and petroleum ether in the mixture was 1:60, and about 16.3 mmol of the precursor compound was obtained.
[0049] b. Target product synthesis:
...
Embodiment 2
[0054] A preparation method of 2,3-disubstituted indole derivatives comprises the following steps:
[0055] a. Precursor compound synthesis:
[0056] In an anhydrous and oxygen-free system, add 20mmol of o-hexynylaniline, 17mmol of trifluoroacetic acid and 40ml of carbon tetrachloride solvent into a 100ml reaction flask, then add 50mmol of triphenylphosphine and 22mmol of triethylamine in an ice-water bath environment Stir for 10 minutes, then heat and reflux for 3 hours, stop the reaction, add water to wash several times, then extract and concentrate with ethyl acetate, dry with anhydrous magnesium sulfate, and finally use ethyl acetate with a volume ratio of 1: (40-80) The mixture of ester and petroleum ether was used as the eluent for column chromatography separation and purification. The volume ratio of ethyl acetate to petroleum ether in the mixture was 1:60, and about 16.5 mmol of the precursor compound was obtained.
[0057] b. Target product synthesis:
[0058] Put 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com