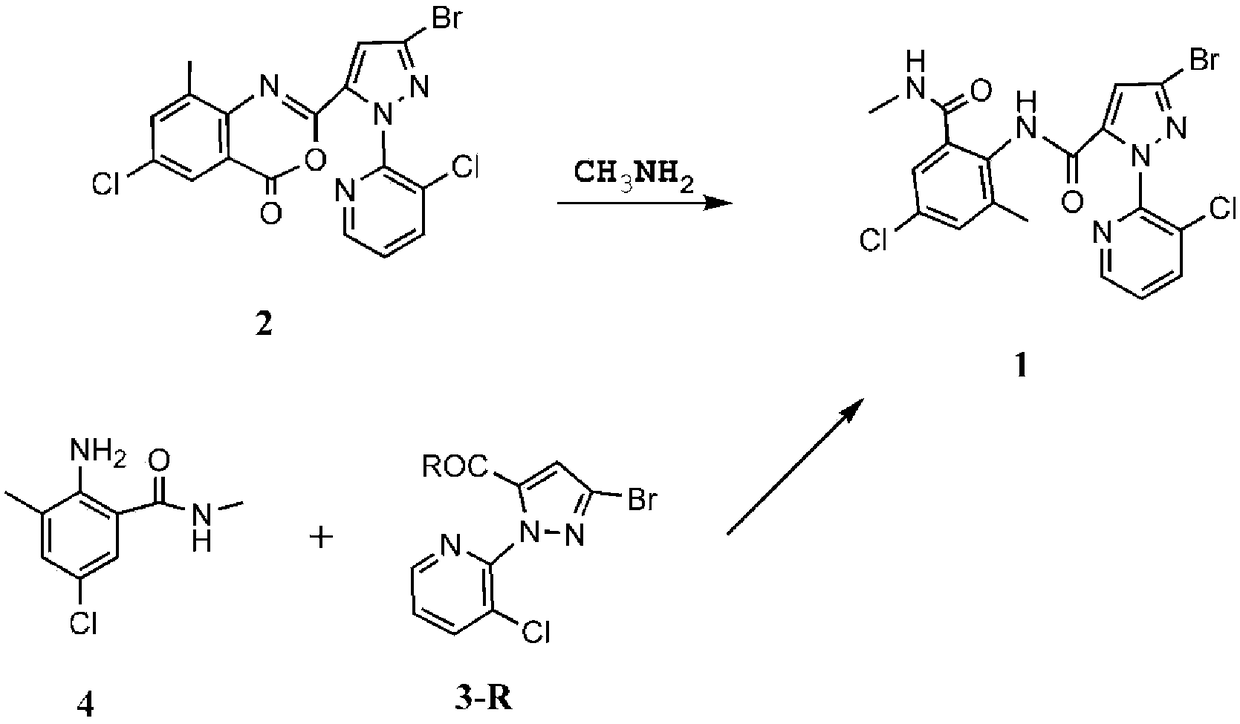
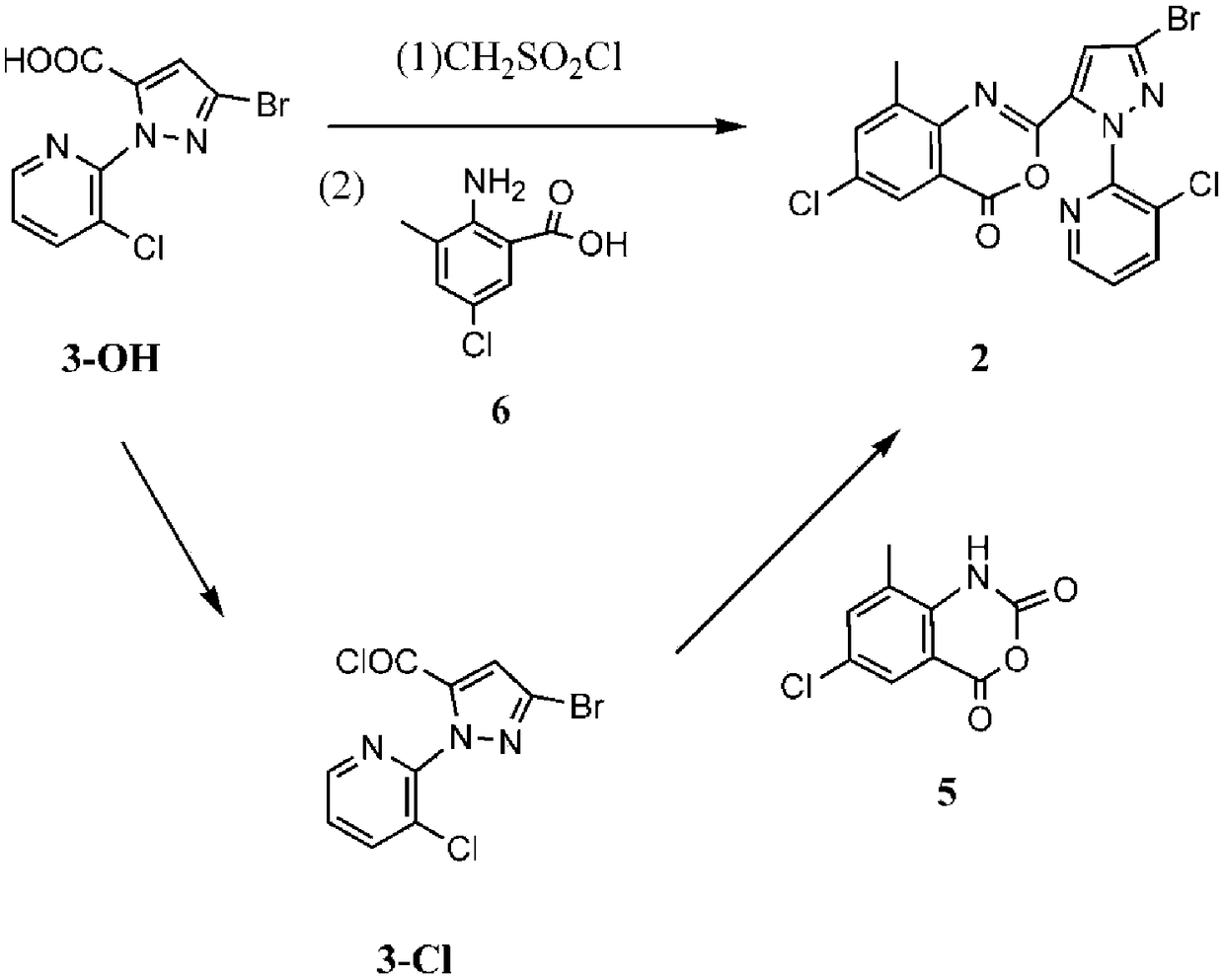
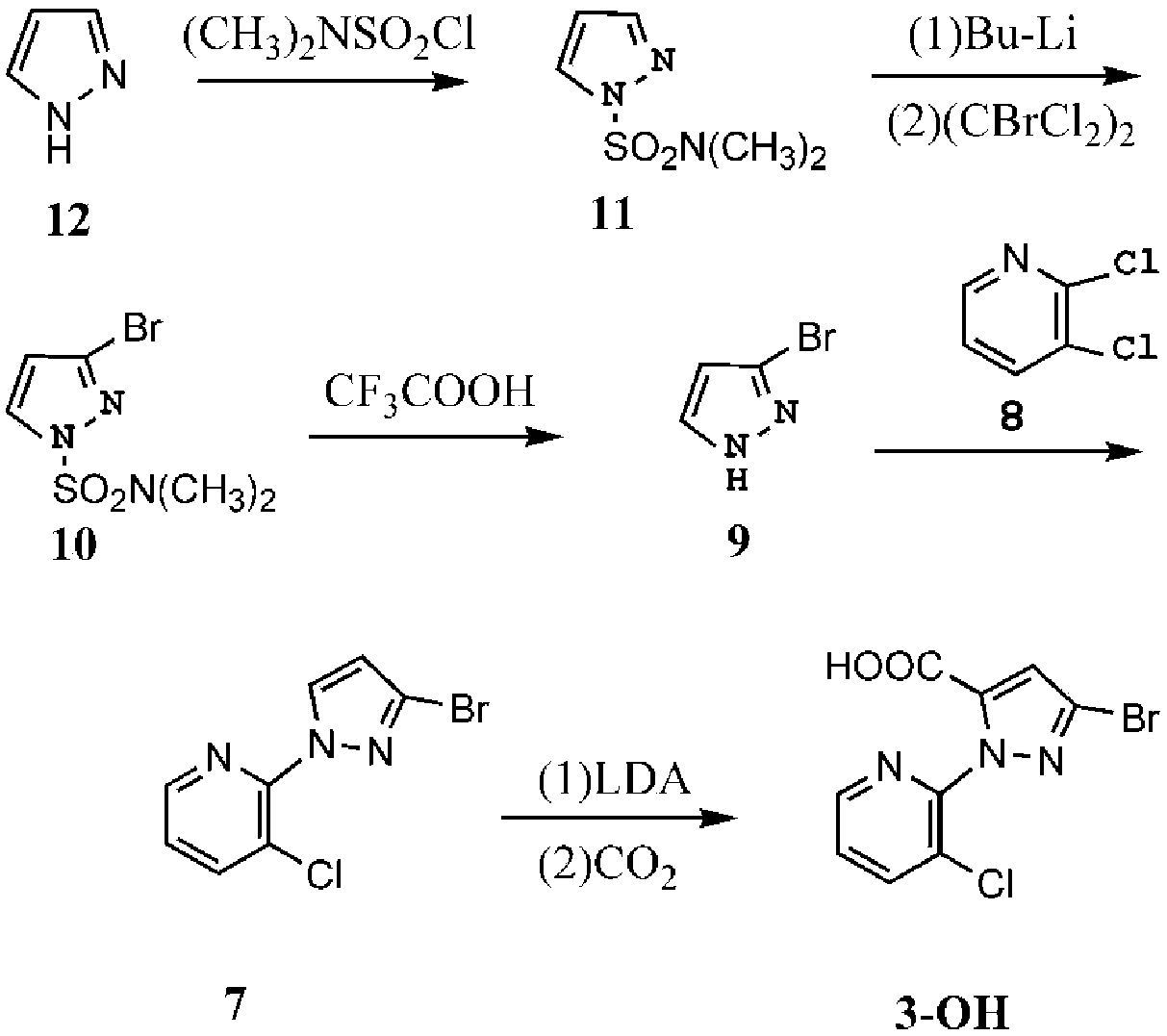
Method for synthesizing chlorantraniliprole
A technology of chlorantraniliprole and synthetic method, applied in the field of organic synthesis, can solve problems such as low reaction yield and harsh reaction conditions, and achieve the effects of easy separation and purification, less by-products, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Synthesis of S1.N-methyl-3-methyl-2-amino-benzamide (17)
[0050]
[0051] Take a 100mL three-necked flask, suspend 0.45g of 2-amino-3-methylbenzoic acid (16) in 30mL of distilled CH 2 Cl 2 In, add 0.4g HOBt (1-hydroxybenzotriazole). 0.84 g of 30% methylamine alcohol solution was added in an ice bath, and 0.86 g of EDCI was added with stirring in an ice bath. After the feeding was completed, the mixture was stirred and reacted in an ice bath for about 30 minutes. Then remove the ice bath, and leave overnight at room temperature. After the reaction, the obtained reaction was washed three times with 30 mL of water, dichloromethane was distilled off under reduced pressure to obtain a white solid, which was weighed to obtain 0.45 g after drying, with a yield of 90%.
[0052] Synthesis of S2.N-methyl-3-methyl-2-amino-5-chloro-benzamide (4)
[0053]
[0054] Take a 100mL three-necked flask, dissolve 0.45g of N-methyl-3-methyl-2-amino-benzamide (17) in 20mL of aceto...
Embodiment 2
[0056] S1. Synthesis of 3-amino-5-methylpyrazole (19)
[0057]
[0058] Put 2.46g of 3-aminocrotononitrile (18) and 20mL of 25% hydrazine hydrate mixture into a 50mL three-necked flask. Heat to reflux for reaction. After the reaction, excess hydrazine hydrate was vacuum-dried. After distillation of the residue, 1.9 g of 3-amino-5-methylpyrazole (19) were obtained, yield 65%.
[0059] S2. Synthesis of 3-methyl-5-bromopyrazole (20)
[0060]
[0061] Mix 1.9g of 3-amino-5-methylpyrazole (19), 15mL of concentrated hydrobromic acid, and 2.8g of cuprous bromide in a 100mL three-necked flask, and heat to 70°C. Dissolve about 1.5g of sodium nitrite in 5mL of water, and slowly drop it into a three-necked flask using a constant pressure funnel. After the addition was complete, the reaction solution was stirred at 70°C for 30 min, then cooled to room temperature, and 20 mL of THF (tetrahydrofuran) and 20 mL of water were added. It was extracted three times with 30 mL of diethy...
Embodiment 3
[0069] S1. Synthesis of Chlorantraniliprole (1)
[0070]
[0071] Dissolve 1 eq 1-(3-chloro-2-pyridyl)-3-bromo-1H-5-pyrazolecarboxylic acid (3-OH) in an appropriate amount of redistilled CH 2 Cl 2 In, add 1.3eq HOBt. 1eq N-methyl-3-methyl-2-amino-5-chloro-benzamide (4) was added in an ice bath, and 1.3eq EDCI was added with stirring in an ice bath. After the feeding was completed, the mixture was stirred and reacted in an ice bath for about 30 minutes. Then remove the ice bath, and leave overnight at room temperature. After the reaction, the obtained reaction was washed three times with 30 mL of water, and dichloromethane was distilled off under reduced pressure to obtain chlorantraniliprole. The yield was 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com