Aza aromatic compound used as blue fluorescent material and application
A technology of aromatic compounds and blue fluorescence, applied in the field of aza aromatic compounds, can solve the problems such as the decrease of luminous efficiency, and achieve the effect of high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
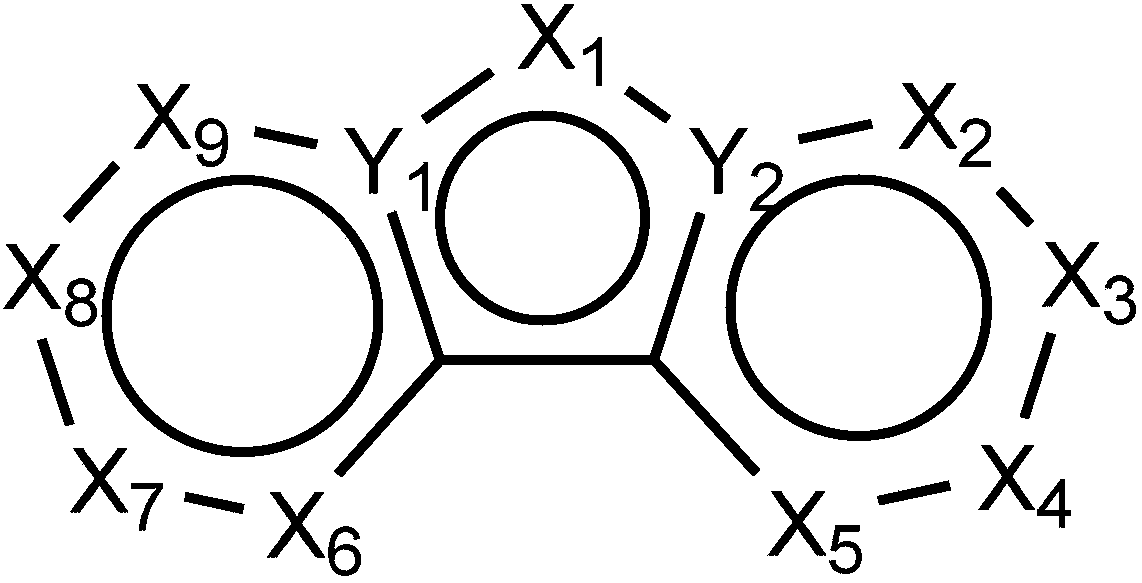
[0032] The synthesis of compound 14, the reaction equation is as follows:
[0033]
[0034]
[0035] The specific preparation method is as follows:
[0036](1) In a 100mL there-necked flask, add 1-(3-bromophenyl)-8 methylnaphthalene (R1a) 1.31g (5mmol), 2-pyridineboronic acid (Re2) 1.48g (12mmol, potassium carbonate 1.76g ( 20mmol), toluene 40g and deionized water 15g, nitrogen protection, add tetrakistriphenylphosphine palladium 0.1g, heat up to 65 ℃, reflux for 18h, stop the reaction, cool down, separate liquid, collect organic phase, remove solvent, obtain intermediate body int1a;
[0037] (2) Under nitrogen protection, zinc bromide (ZnBr 2 ) 1.11g (0.5mmol), MnBr(CO) 5 69.0 mg (0.25 mmol), 50 mL of dichloroethane (DCE), INT1a obtained in step (1), 6-methyl-benzofuran-5-carbaldehyde (R3a) 1.6 g (10 mmol), Me 2 7.5mL (7.5mmol) of Zn was added to a 250mL Schlenk bottle in turn; then the temperature was raised to 60°C and stirred for 12h; the reaction mixture was di...
Embodiment 2
[0040] The synthesis of compound 23, the reaction equation is as follows:
[0041]
[0042] The specific preparation method is as follows:
[0043] The preparation method is the same as in Example 1, except that 2-(3'-bromo-1,1'-biphenyl)naphthalene (R1b) is used in step (1) to react with 2-pyridineboronic acid (Re2); 2), use 1,1'-biphenyl-4-carbaldehyde (R3b) to react with INT1b; the product Pb is a light yellow solid to obtain compound 23.
Embodiment 3
[0045] The synthesis of compound 29, the reaction equation is as follows:
[0046]
[0047] The specific preparation method is as follows:
[0048] It is the same as step (2) (3) of the preparation method of Example 1, except that 2-phenylpyridine (R1c) is used in step (1) to react with 4-benzofuran-4-yl-benzaldehyde (R2c) to generate INT1c INT1c generates light yellow solid product Pc through step (2) to obtain compound 29.
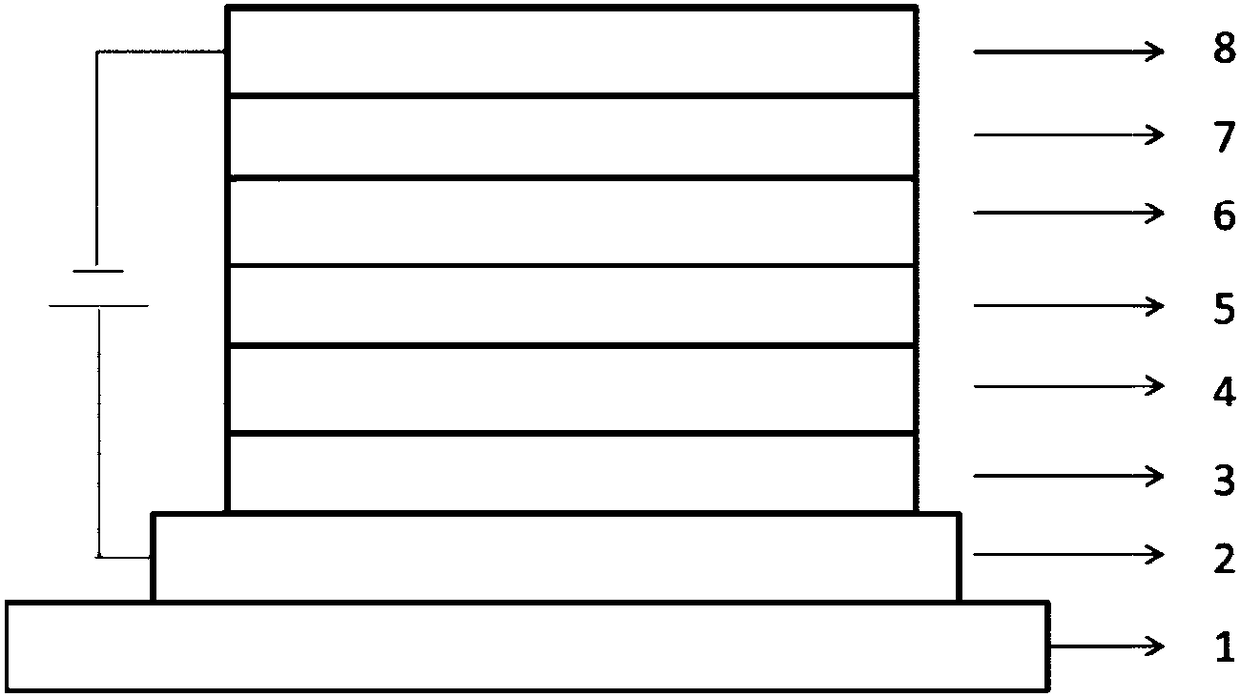
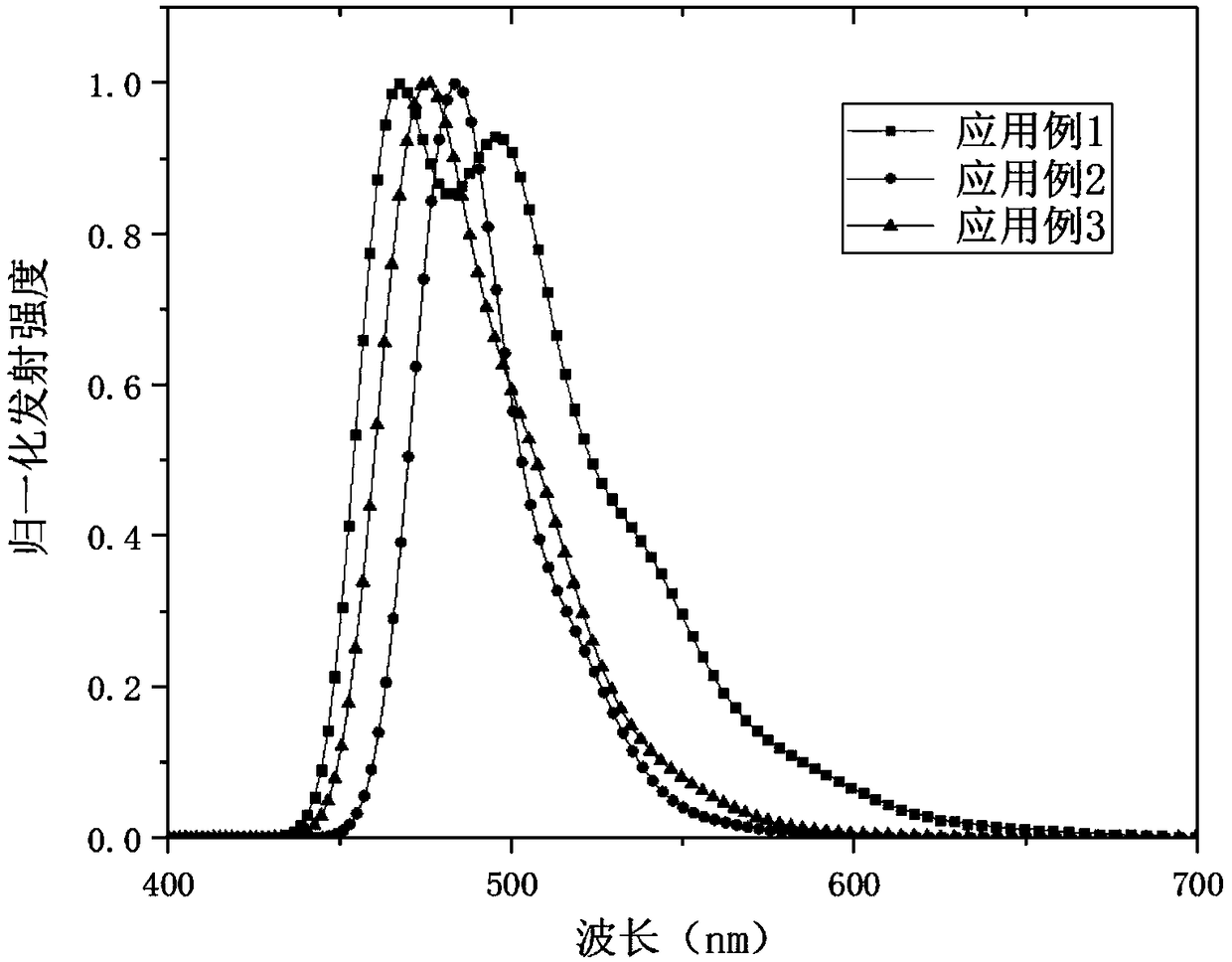
[0049] like figure 1 As shown, the structure of the organic electroluminescent device (OLED) includes a glass substrate 1, an anode layer 2, a hole injection layer 3, a hole transport layer 4, a light-emitting layer 5, an electron transport layer 6, an electron injection layer and an layer 7 and cathode layer 8. The aza aromatic compound prepared by the present invention is applied in the light-emitting layer of the OLED, and Table 1 shows the composition of each layer of the OLED in application examples 1-3.
[0050] Table 1
[0051]
[0052]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum luminescence wavelength | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com