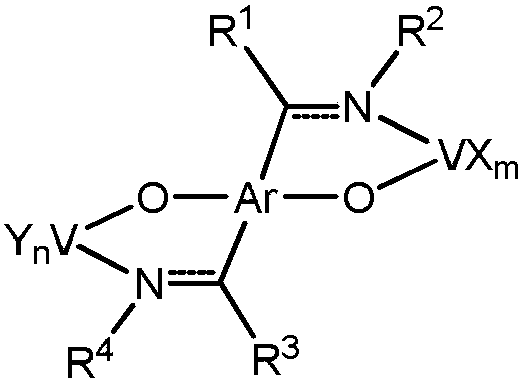
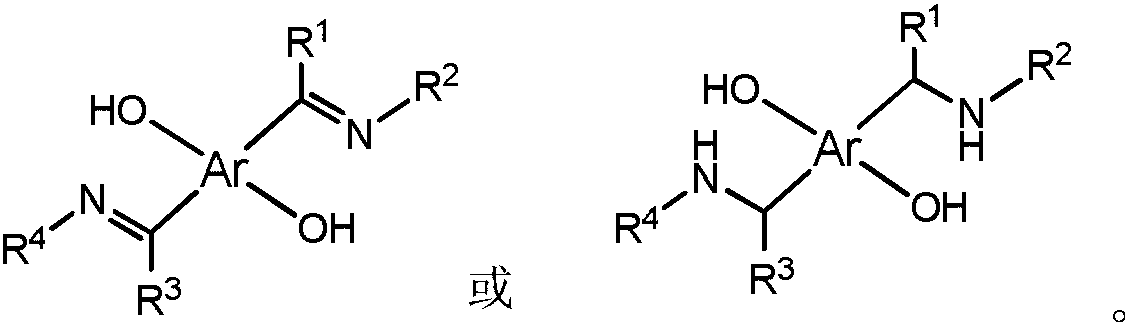
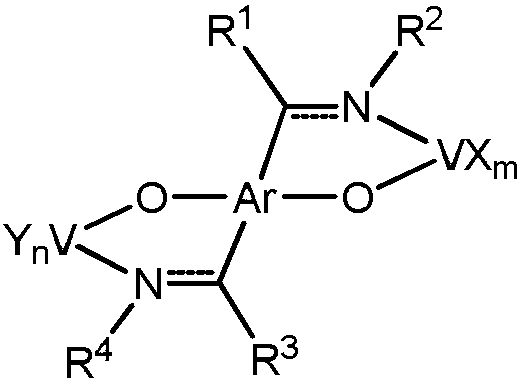
Non-metallocene bidentate double-vanadium complex as well as preparation method and application thereof
A vanadium complex, non-locene technology, applied in the field of non-locene bidentate double vanadium complexes and preparation, can solve the problems of increasing the molecular weight of the polymer, broadening the molecular weight distribution, asymmetric bimetallic structure, etc. The effect of range, good stability and low catalyst cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of bidentate double vanadium complex V1
[0032]
[0033] Add 1 mmol of ligand (L1) to the Schlenk bottle, and then evacuated nitrogen for three times, and added 20 ml of tetrahydrofuran to dissolve the ligand; at 0 °C, the ligand solution was introduced into 10 ml of tetrahydrofuran suspension dissolved with 2 mmol of KH, and the temperature was raised to The reaction was stirred at room temperature for 5 hours; 2 mmol of VCl was added to another schlenk bottle 3 (THF) 3 and 10 ml of tetrahydrofuran; introduce the potassium salt solution of the ligand into VCl at -78 °C 3 (THF)3 In the solution, the temperature was slowly raised to 40 °C and reacted for 36 h, then filtered, concentrated under reduced pressure, slowly added n-hexane to cover the upper layer of the solution, and allowed to stand to obtain a black solid powder with a yield of 76%. Elemental analysis: Theoretical (%): C, 58.64; H, 5.95; N, 2.85; Tested (%): C, 58.32; H, 5.43; N, 3.06.
Embodiment 2
[0035] Synthesis of bidentate double vanadium complex V2
[0036]
[0037] Add 1mmol ligand L2 in Schlenk bottle, replace with nitrogen three times, add 20ml dichloromethane to dissolve the ligand, add dissolved 1mmol VO (O i Pr) 3 10ml of dichloromethane solution, stirred at room temperature for 12 hours, then added 1mmol of o-methylimino vanadium trichloride in dichloromethane solution, then added dropwise 0.2ml of triethylamine, stirred at room temperature for 12 hours, The solution was concentrated to 20 ml, the upper layer was covered with hexane, and after standing, a black solid powder was obtained with a yield of 58%. Elemental analysis: Theoretical value (%): C, 58.44; H, 6.58; N, 4.99; Test value (%): 58.43; H, 6.68; N, 4.55.
Embodiment 3-11
[0039] Synthesis of Bidentate Bivanadium Complexes V3-V11
[0040]
[0041] L3, V3: R=H; L4, V4: R=2- i Pr; L5, V5: R=2,6- i Pr 2 ; L6, V6: R=2-F; L7, V7: R=3-F; L8, V8: R=4-F; L9, V9: R=2,4-F 2 ;L10,V10: R=3,4,5-F 3 ; L11, V11: R=2,3,4,5,6-F 5
[0042] V3
[0043] room temperature N 2 Under atmosphere, add VCl to the dry Schlenk bottle 3 (THF) 3 (0.26g, 0.7mmol), dissolve in 10ml of tetrahydrofuran, add Schiff base bidentate ligand L3 (0.25g, 0.35mmol) and 10ml of tetrahydrofuran to another Schlenk bottle, stir to dissolve the ligand, then slowly dissolve the ligand The tetrahydrofuran solution was introduced into VCl 3 (THF) 3 tetrahydrofuran solution, and after stirring for 10 min, Et was added dropwise to the mixed solution. 3 N (0.1 ml, 0.73 mmol), Et was added 3 The solution turned black after N. Stir at room temperature for 12 h, remove the solvent in vacuo to obtain a black solid, add 5 ml of tetrahydrofuran to dissolve in the glove box, filter out the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com