A jelly-like mouthwash with anti-caries, antibacterial and strong root tooth-fixing effects
A tooth-strengthening and mouthwash technology, which is applied to medical preparations containing active ingredients, antibacterial drugs, organic chemistry, etc., can solve the problems of caries-free prevention and tooth-strengthening, and achieve enhanced anti-caries effect, The preparation process is simple and convenient, and the effect of improving the anti-inflammatory and analgesic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
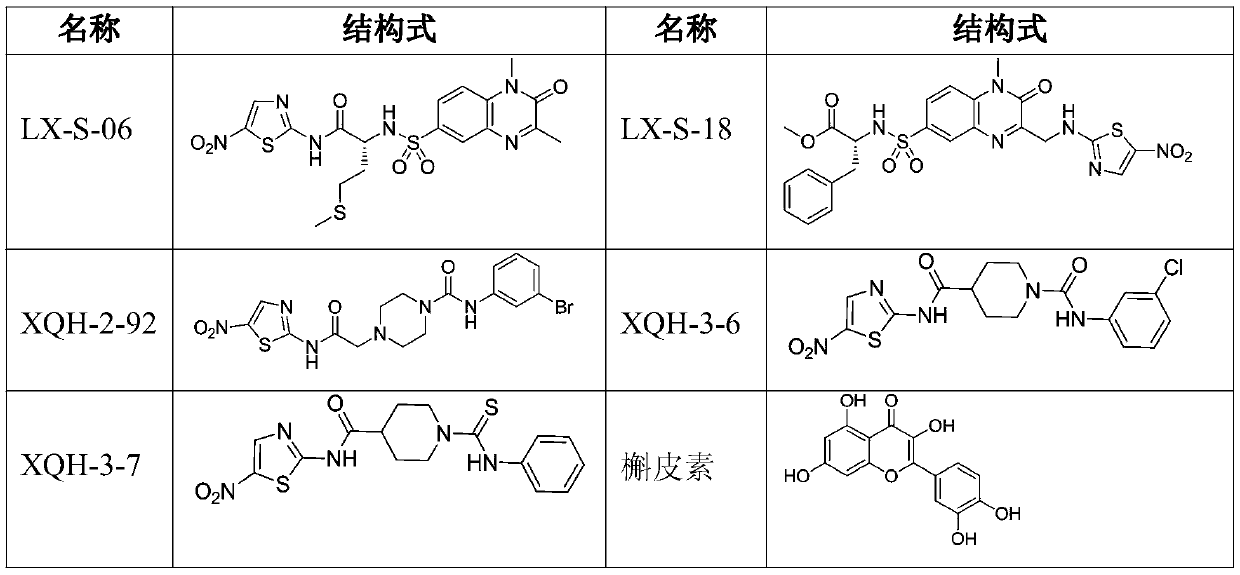
[0037] Example 1: (S)-2-(1,3-dimethyl-2-oxo-1,2-dihydroquinoxaline-6-sulfonamido)-3-(methylthio)-N- Preparation of (5-nitrothiazol-2-yl) propionamide (compound LX-S-06)
[0038]
[0039] Preparation of intermediate 3-methylquinoxalin-2(1H)-one (1)
[0040] Weigh 10.81g (0.10mol) of o-phenylenediamine into a 500mL round bottom flask, add 250mL of absolute ethanol, and stir until completely dissolved to obtain a light yellow solution. 12.77 g (0.11 mol) of ethyl pyruvate was added dropwise to the solution with stirring at room temperature. After about 10 minutes, a large amount of solids were generated instantaneously, and the reaction was continued for 5 hours, and the reaction ended. After filtration, the solid was collected and recrystallized with ethanol. After vacuum drying, a light white cotton wool-like product was obtained. The yield was 90.4%, m.p.=241.0-242.7°C.
[0041] Preparation of intermediate 1,3-dimethylquinoxalin-2(1H)-one (2)
[0042] Weigh 8.01g (0.05mo...
Embodiment 2
[0051] Example 2: (R)-methyl(2-(1-methyl-3-(((5-nitrothiazole-2-)amino)methyl)-2-oxo-1,2-dihydroquinone Preparation of oxaline-6-sulfonamido)-3-phenylpropionyl) carbamate (compound LX-S-18)
[0052]
[0053]Intermediate (R)-methyl 2-(1,3-dimethyl-2-oxo-1,2-dihydroquinoxaline-6-sulfonamido)-3-phenylpropanoic acid methyl ester (6 ) preparation
[0054] The preparation method of the intermediate (6) is the same as that of the intermediate (4) in Example 1, and the yield is 69%. M.p=151.0~153.0℃, 1 H-NMR (DMSO-d 6 ,400MHz,ppm):δ2.473(s,3H,N=CCH 3 ), 2.751 (dd, J=9.0, 13.8Hz, 1H, CH 2 ), 2.93 (dd, J=6.0, 13.8Hz, 1H, CH 2 ),3.34(s,3H,OCH 3 C=O),3.63(s,3H,NCH 3 ), 4.08(d, J=7.8Hz, 1H, CH), 7.05-7.14(m, 5H, 5ArH), 7.57(d, J=9.0Hz, 1H, ArH), 7.69(dd, J=2.4, 9.0 Hz,1H,ArH),7.85(d,J=2.4Hz,1H,ArH),8.63(d,J=9.0Hz,1H,NH).ESI-MS:416.7[M+H] + .
[0055] Intermediate (R)-Methyl 2-(3-bromomethyl)-1-methyl-2-oxo-1,2-dihydroquinoxaline-6-sulfonamido)-3-phenylpropionic acid Preparat...
Embodiment 3
[0059] Embodiment three: the antibacterial activity test of compound LX-S-06
[0060] Preparation of Streptococcus mutans
[0061] (1) The medium for cultivating Streptococcus mutans is Brain heart infusion medium (brand OXOID, product number CM1135), the main components of which are Brain infusion solids 12.5g / L, Beef heartinfusion solids 5.0g / L , Proteose peptone 10.0g / L, Glucose 2.0g / L, Sodiumchloride 5.0g / L, Di-sodium phosphate 2.5g / L, pH 7.4±0.2. If solid medium is required, add 1.5% agar powder. Sterilize with damp heat at 115°C for 30 minutes, and cool down for use.
[0062] (2) The culture medium for Streptococcus mutans biofilm is brain heart infusion-sucrose medium. The preparation method is to make 20% stock solution of sucrose, filter and sterilize it with a 0.22 μm sterile filter, and add 1% sucrose to the brain-heart infusion medium.
[0063] (3) Streptococcus mutans type strain UA159 and clinical strain UA246 were inoculated on the solid medium of brain hear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com