A kind of phosphopeptide solid-phase extraction column and its preparation and application
A technology of solid-phase extraction cartridges and phosphopeptides, which is applied in chemical instruments and methods, other chemical processes, instruments, etc., can solve the problems of inapplicable analysis of trace samples, long time consumption, etc., to reduce the complexity of interference and improve the preparation method Simple, highly specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
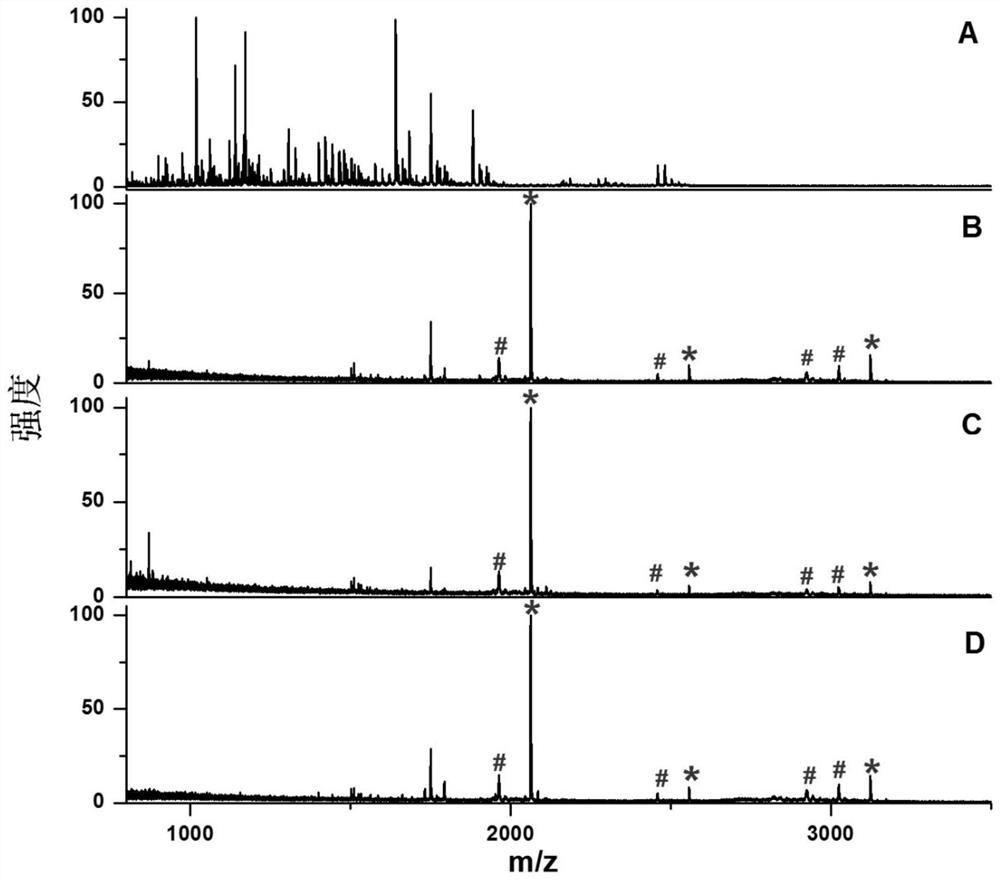
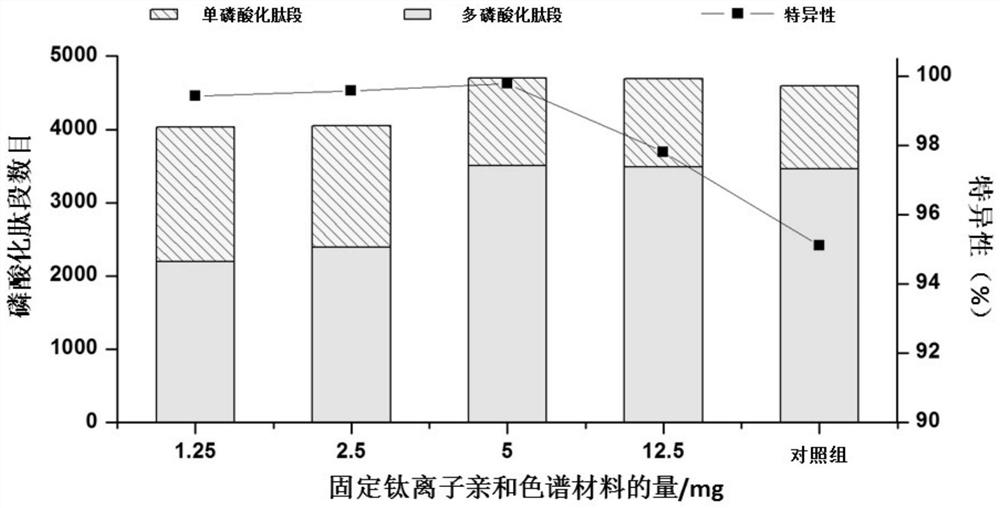
[0028]The phosphopeptide solid phase extraction cartridge is used for the enrichment of phosphopeptides in the enzymatic hydrolysate of the mixture of bovine serum albumin and β-casein:
[0029](1) Disperse 1 g of polyvinyl alcohol into 120 mL of deionized water to obtain a dispersion medium;
[0030](2) Mix 18 mL of toluene, 18 mL of ethylene glycol dimethacrylate, 20 mL of glycidyl methacrylate and 0.12 g of benzoyl peroxide to obtain a monomer phase;
[0031](3) Add the monomer phase of step (2) to the dispersion medium of step (1) and heat and stir at 70° C. for 12 hours to obtain polymer microspheres;
[0032](4) Reacting the polymer microspheres obtained in step (3) with 150 mL of ethylenediamine to obtain polymer microspheres with amino groups on the surface;
[0033](5) Reacting the polymer microspheres with amino groups on the surface obtained in step (4) with 5 mL phosphoric acid, and then reacting with 100 mM titanium sulfate at 25° C. for 16 hours to prepare a fixed titanium ion affini...
Embodiment 2
[0044]The phosphopeptide solid phase extraction cartridge is used for the enrichment of phosphopeptides in the HeLa hydrolysis solution:
[0045](1) Disperse 1 g of polyvinyl alcohol into 120 mL of deionized water to obtain a dispersion medium;
[0046](2) Mix 18 mL of toluene, 18 mL of ethylene glycol dimethacrylate, 20 mL of glycidyl methacrylate and 0.12 g of benzoyl peroxide to obtain a monomer phase;
[0047](3) Add the monomer phase of step (2) to the dispersion medium of step (1) and heat and stir at 70° C. for 12 hours to obtain polymer microspheres;
[0048](4) Reacting the polymer microspheres obtained in step (3) with 150 mL of ethylenediamine to obtain polymer microspheres with amino groups on the surface;
[0049](5) Reacting the polymer microspheres with amino groups on the surface obtained in step (4) with 5 mL phosphoric acid, and then reacting with 100 mM titanium sulfate at 25° C. for 16 hours to prepare a fixed titanium ion affinity chromatography filler;
[0050](6) Fill the sieve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com