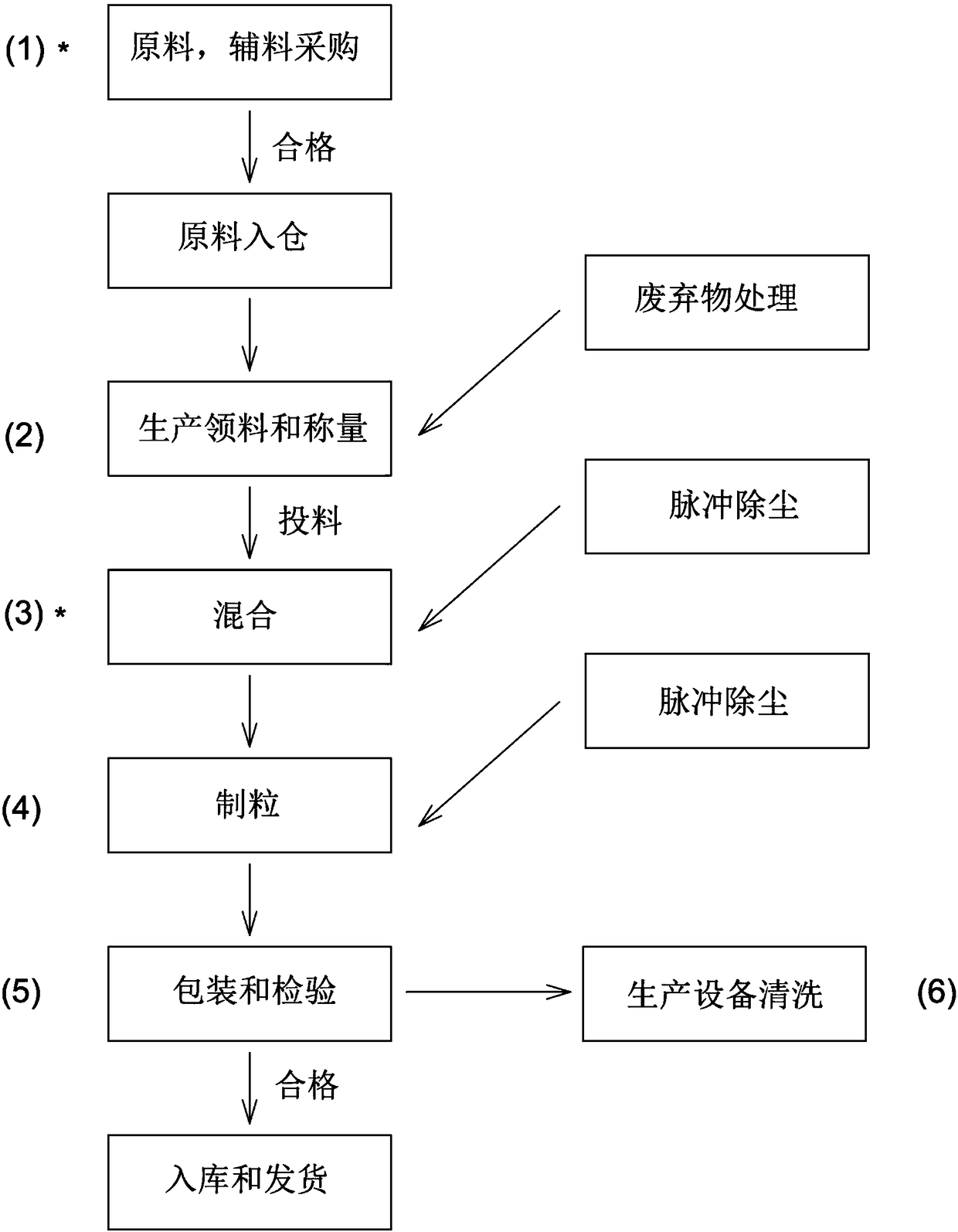
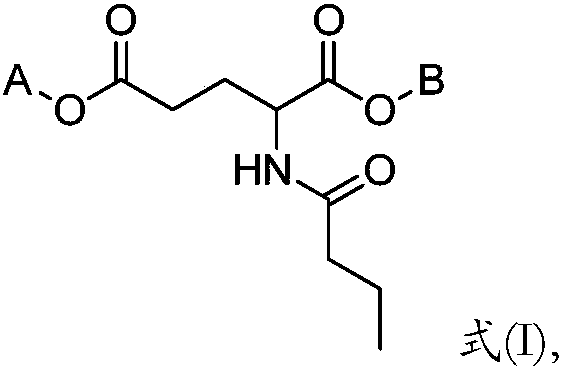
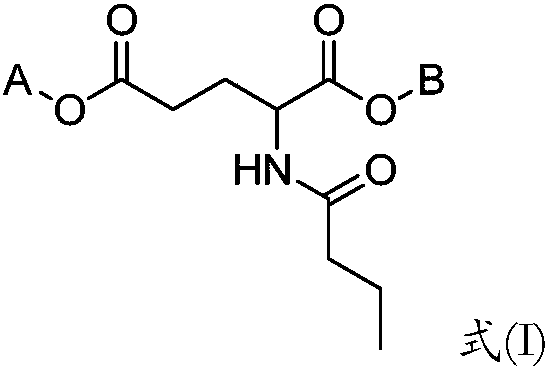
Butyryl glutamic acid derivative as well as composition and application thereof
A technology of butyryl glutamic acid and derivatives, which is applied in the field of preparation of animal feed additives or feed, and can solve the problems of mutagenesis, urinary tract disorders, and large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Embodiment 1 butyryl glutamic acid
[0112]
[0113] In a 2L three-necked flask, 200.34g (1.36mol, 1eq) of L-glutamic acid and 850mL (136.00g, 3.40mol, 2.5eq) of 4M NaOH aqueous solution were added successively, and after stirring at room temperature for 0.5 hours, 215.44g of butyric anhydride ( 1.36mol, 1eq.), and then added dropwise 4M NaOH aqueous solution to adjust the pH to 8-9, and TLC monitored the reaction to the end. Concentrated hydrochloric acid was added dropwise to the reaction solution to adjust the pH to 2-3, extracted with ethyl acetate (1L×3), and the organic phases were combined. The organic phase was washed with saturated brine (1L×3), dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain the crude product as a colorless transparent liquid, which was cooled to 0°C for crystallization, filtered with suction, and the filter residue was washed with n-heptane (100mL× 3) The product (butyryl glutamic acid) was dried und...
Embodiment 2
[0114] Embodiment 2 N-butyryl-L-glutamic acid diethyl ester
[0115]
[0116] Step 1: Synthesis of L-glutamic acid diethyl ester hydrochloride
[0117]Add 320mL of absolute ethanol (5.48mol, 40eq) to a 1000mL single-necked bottle, cool to minus 10°C, and slowly add 28mL of thionyl chloride (378.92mmol, 2.8eq) dropwise into the reaction system. After the dropwise addition, the reaction solution was stirred and reacted at a constant temperature of minus 10° C. for 1 hour, and then 20.08 g (136.48 mmol, 1 eq) of L-glutamic acid was added to the reaction system. The reaction solution was stirred at room temperature for 2 hours, then heated to 80° C. for reaction, and monitored by TLC until the end point. The reaction liquid was concentrated under reduced pressure to remove the solvent, cooled and crystallized in the refrigerator, added ethyl acetate (150mL×3) to wash, separated by suction filtration, and dried to obtain the product (diethyl L-glutamate hydrochloride) as a whit...
Embodiment 3
[0120] Example 3 N-butyryl-L-glutamic acid bis-n-butyl ester
[0121]
[0122] Step 1: Synthesis of L-glutamic acid bis-n-butyl ester hydrochloride
[0123] Add 403.03g (5.44mol, 40eq) of n-butanol to a 1000mL single-necked bottle, cool to minus 10°C, and slowly add 45.28g (380.62mmol, 2.8eq) of thionyl chloride dropwise into the reaction system. After the dropwise addition, the reaction solution was stirred and reacted at a constant temperature of minus 10° C. for 1 hour, and then 20.00 g (135.93 mmol, 1 eq) of L-glutamic acid was added to the reaction system. The reaction solution was stirred at room temperature for 2 hours, then heated to 85° C. for reaction, and monitored by TLC until the end point. The reaction solution was concentrated under reduced pressure to remove the solvent, cooled and crystallized in the refrigerator, added ethyl acetate (150mL×3) to wash, separated by suction filtration, and dried to obtain the product (L-glutamic acid bis-n-butyl hydrochlori...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com