Interleukin 39 specific antagonistic protein and application thereof in treating autoimmune diseases
A specific, interleukin-based technology, applied in the direction of allergic diseases, bone diseases, immunoglobulin, etc., can solve the problems of long course of disease and low cure rate, and achieve obvious therapeutic effects, alleviate pathological changes, and significant therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]Example 1 Obtaining of 23R-gp130 protein coding gene
[0023] A fusion cDNA fragment encoding IL-23R receptor extracellular domain, Linker peptide (GGGGGSS), gp130 receptor extracellular domain, and Linker peptide (GGGGGSS) was obtained by using the method of whole gene synthesis, and the 5' end contains human Il -2 Signal peptide coding sequence. The synthesized DNA sequence was inserted into the recombinant plasmid pSBS1-Cloning Vector to obtain the pSBS1-23R-gp130 plasmid. The synthetic sequence is shown in SEQ ID No.6.
Embodiment 2
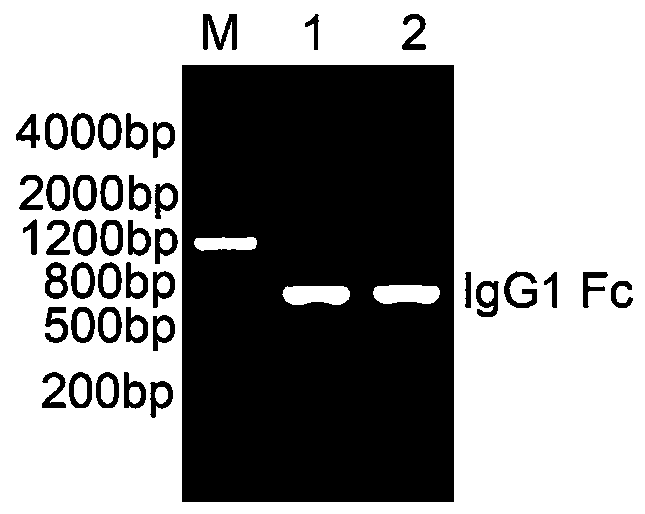
[0024] Example 2 RT-PCR amplification of human IgG1 Fc cDNA fragment
[0025] 1. Extraction of total RNA from human lymphocytes
[0026] Blood sample collection: Use a disposable anticoagulant vacuum blood vessel for blood collection, and the blood collection volume is generally 2ml.
[0027] 2. Isolation of lymphocytes
[0028] ① Extract lymphocytes. Take a 1.5ml sterile enzyme-free centrifuge tube, tube ①, first add 500μl lymphocyte separation solution; another 1.5ml sterile enzyme-free centrifuge tube, a vacuum tube containing 500μl blood sample and 500ul Hank's solution, 1:1 Mix the mixture to a total of 1ml. Then slowly add the mixed blood sample Hank's mixture into tube ① with lymphocyte separation solution. Centrifuge horizontally at 1500 rpm for 15 minutes at room temperature. ②After centrifugation, the liquid in the tube is divided into three layers, the upper layer is plasma and Hank’s solution, and the lower layer is mainly red blood cells and granulocytes. Th...
Embodiment 3
[0042] Example 3 Construction of IL23R-gp130-Fc protein coding gene recombinant plasmid
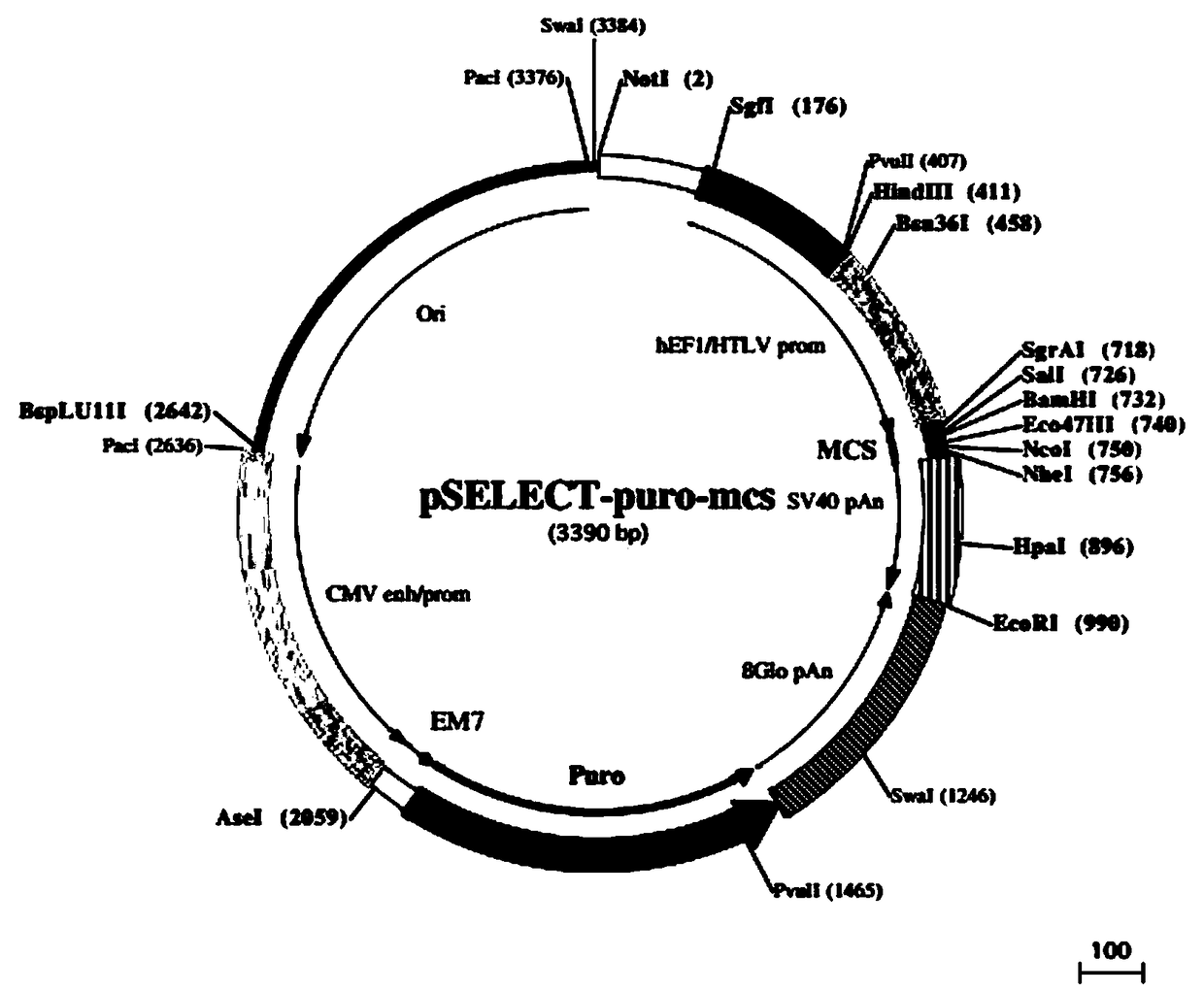
[0043] As mentioned above, the 5' end of the whole gene synthesis fragment 23R-gp130 is designed with a Sal I restriction site, and the 3' end is designed with a BamH I restriction site, so that the cDNA fragment of 23R-gp130 can be directly inserted into eukaryotic expression Vector pSelect-Puro (purchased from Invivogen, USA, see figure 2 ) in the multiple cloning site Sal I / BamH I, and the IgG1 Fc fragment was inserted into the multiple cloning site BamH I / Nhe I to obtain the IL23R-gp130-Fc fusion fragment. See its construction process image 3 ; The finally obtained pSelect-IL23R-gp130-Fc expression vector is as follows Figure 4 shown. Specific steps are as follows:
[0044] 1) Cut the 23R-gp130 sequence from the synthetic recombinant plasmid pSBS1-23R-gp130 with SalⅠ / BamHI, and perform SalⅠ / BamHI double enzyme digestion on the pSelect-Puro vector plasmid at the same time. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Equilibrium dissociation constant | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com