Western blot detection method for phosphoprotein
A phosphorylated protein and phosphorylated protein technology, which is applied in biological testing, material inspection products, etc., can solve the problem of low stoichiometric value of phosphorylated protein phosphorylation sites, slow development of phosphorylated proteomics research, and few analytical and detection methods and other problems, to achieve the effect of low instrument requirements, good effect and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
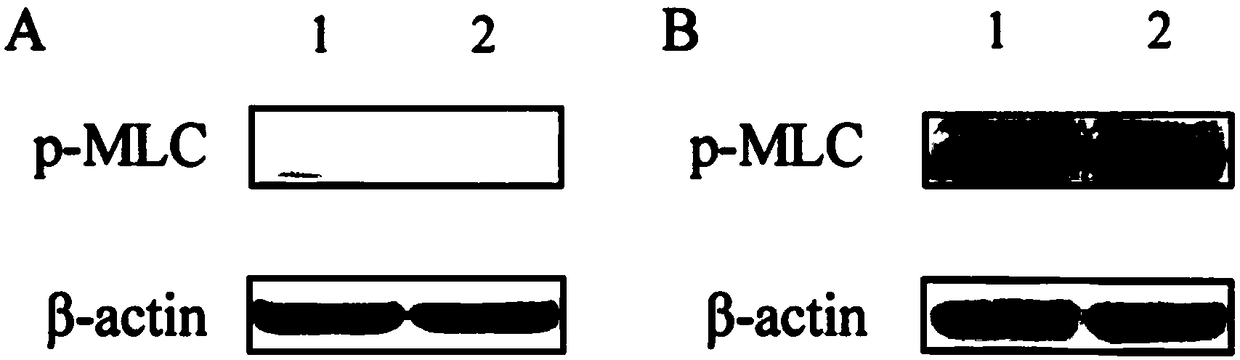
[0022] The phosphorylated protein of myosin light chain (MLC) with a molecular weight of 18kD is proposed to be used as the research object, and the effects of different transfer methods and different antibody dilution ratios on the protein and phosphorylated expression detection are compared.
[0023] Follow the steps below:
[0024] S1. Sample preparation and gel electrophoresis: Add 6 times SDS loading buffer to the phosphorylated protein sample at a ratio of 5:1, mix the sample evenly, and boil it at 100°C for 5 minutes in a dry-type thermostat to make the amino acid side chain of the protein Fully combined with SDS. The denaturation efficiency of DTT and SDS proteins can be improved at 100°C; add 1 times the electrophoresis buffer to the electrophoresis device, add the pre-stained indicator and phosphorylated protein samples to the sample wells of the gel, and set a constant voltage of 80V when starting electrophoresis. When the indicator band runs into the separating ge...
Embodiment 2
[0033] The phosphorylated myosin light chain was extracted from mouse tracheal smooth muscle cells. The amount of protein loaded was 60 μg. The antibody was p-MLC (ab2480). For the wet transfer method, the operation was performed according to the steps described in Example 1.
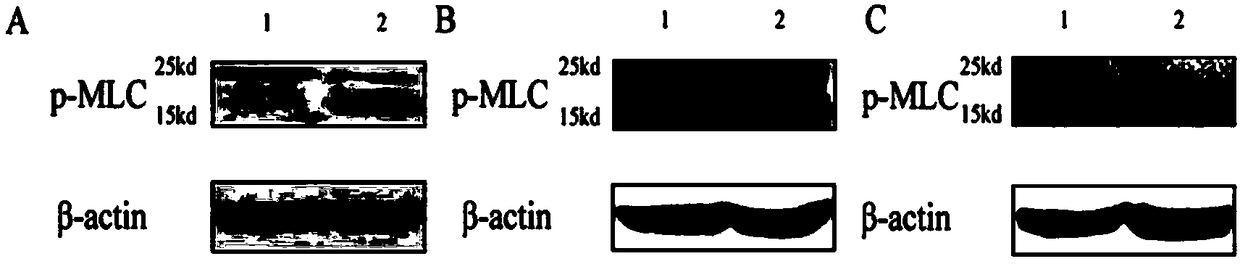
[0034] see figure 2 The comparison chart of the detection results of p-MLC protein expression with different antibody dilution ratios shown shows that: when the antibody dilution ratio is 1:3000, non-specific bands appear with a molecular weight of 25kD ( figure 2 A); When the antibody dilution ratio is reduced to 1:5000, there are no non-specific bands, and the protein signal effect is better, and the average gray value is 59563 ( figure 2 B) When the antibody dilution ratio is 1:10000, there is also no non-specific band, but the band appears diffuse and the background is high, and the average gray value is 50387 ( figure 2 C).
[0035] In the immunoblotting detection of phosphorylated proteins,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com