Aptamer-molecular imprinting fluorescent sensor having double specific recognition on kanamycin as well as preparation method and application of sensor
A fluorescent sensor, kanamycin technology, applied in chemical instruments and methods, fluorescence/phosphorescence, instruments, etc., can solve the problems of aptamer-molecularly imprinted fluorescent sensing that have not been reported, and achieve enhanced dual recognition effect, Good selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
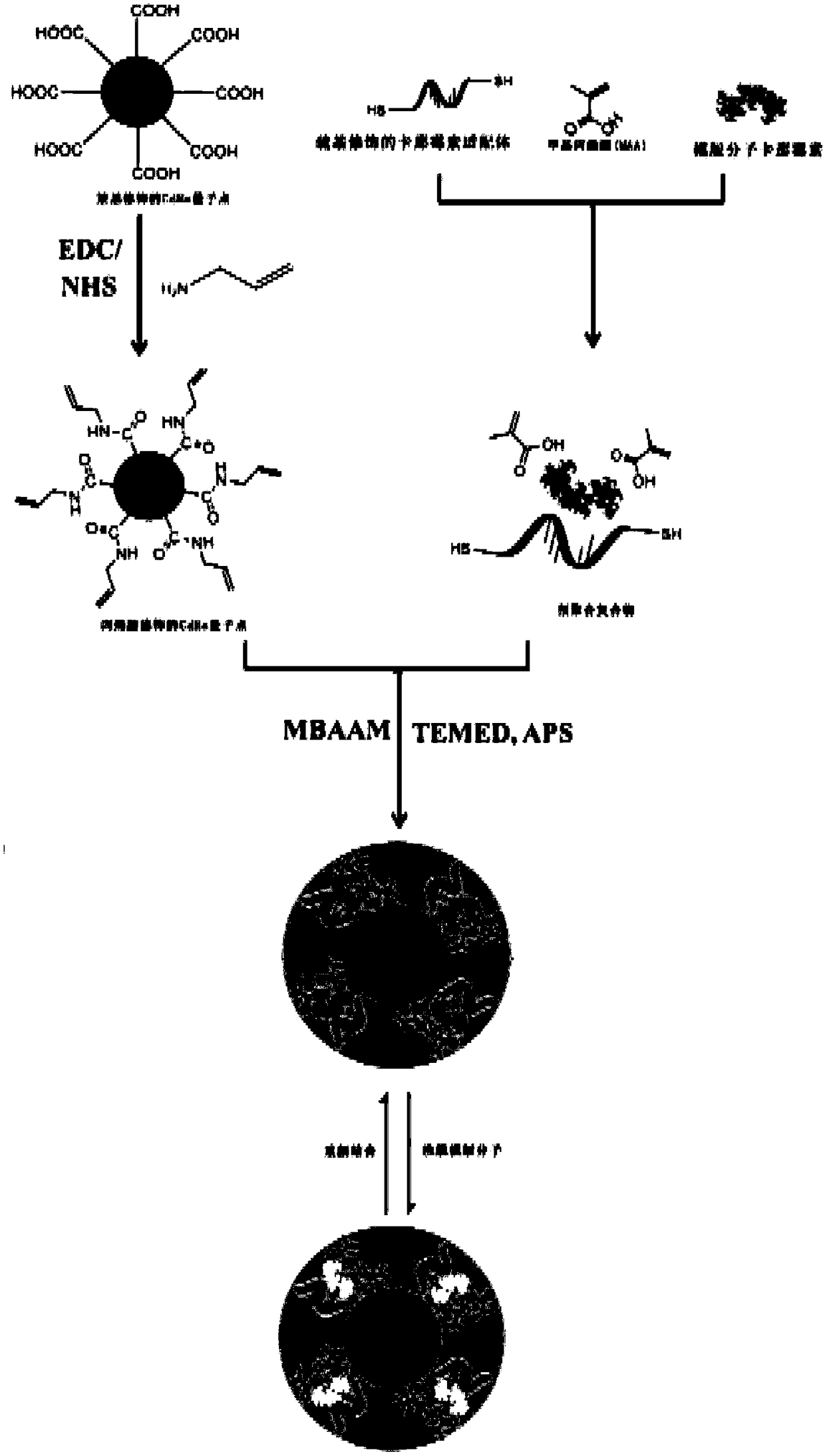
[0063] like figure 1 As shown, the present invention provides a method for preparing an aptamer-molecularly imprinted fluorescent sensor with dual specific recognition for kanamycin, comprising the following steps. First, the template molecule kanamycin, sulfhydryl-modified Mycin aptamer, methacrylic acid to form a pre-polymerization complex; then, the aptamer-quantum dot complex is formed by the pre-polymerization complex, cross-linking agent, allylamine-modified CdSe quantum dots, and initiator; finally, the The template molecule kanamycin was eluted from the aptamer-quantum dot complex, and an aptamer-molecularly imprinted fluorescent sensor with dual specific recognition for kanamycin was obtained.
[0064] The present invention also provides the aptamer-molecular imprinted fluorescence sensor with dual specific recognition for kanamycin prepared by the above preparation method and its application in detecting the content of kanamycin in a sample.
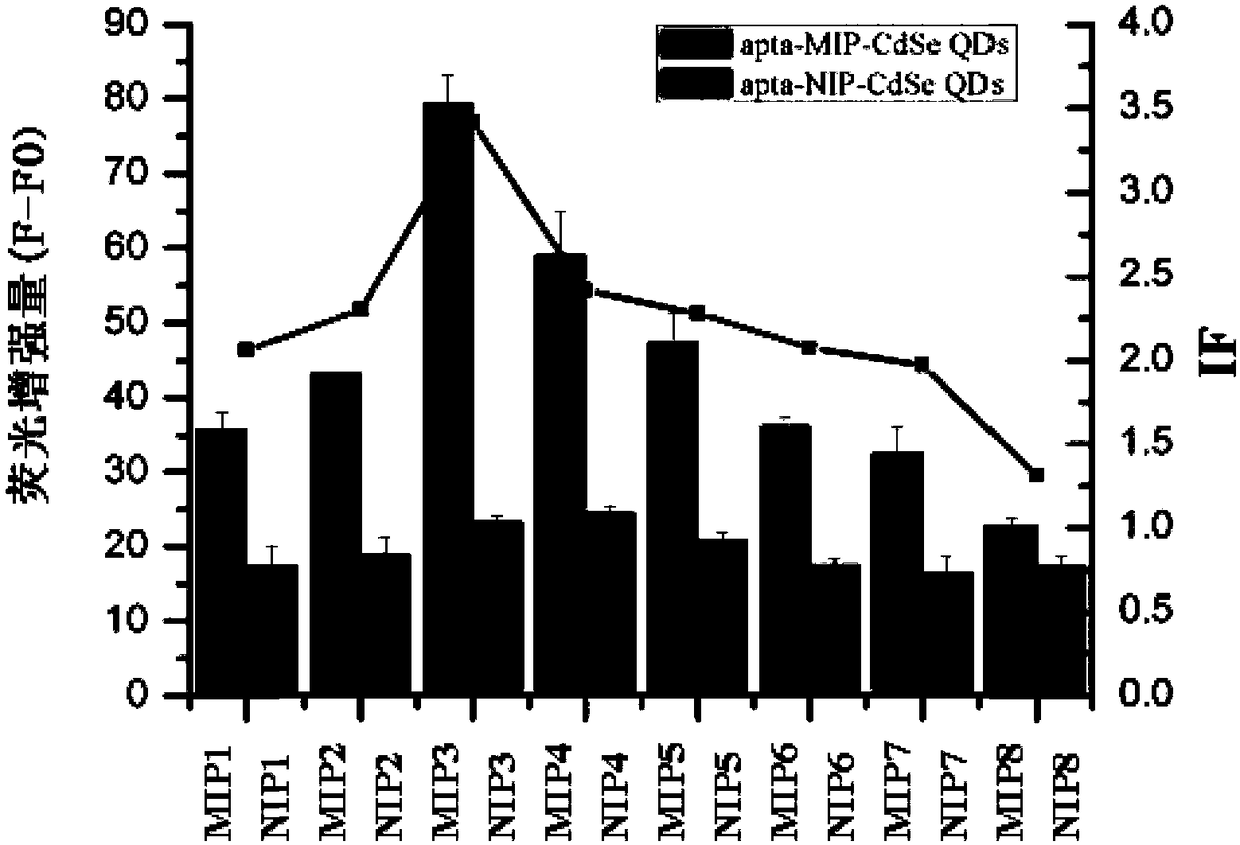
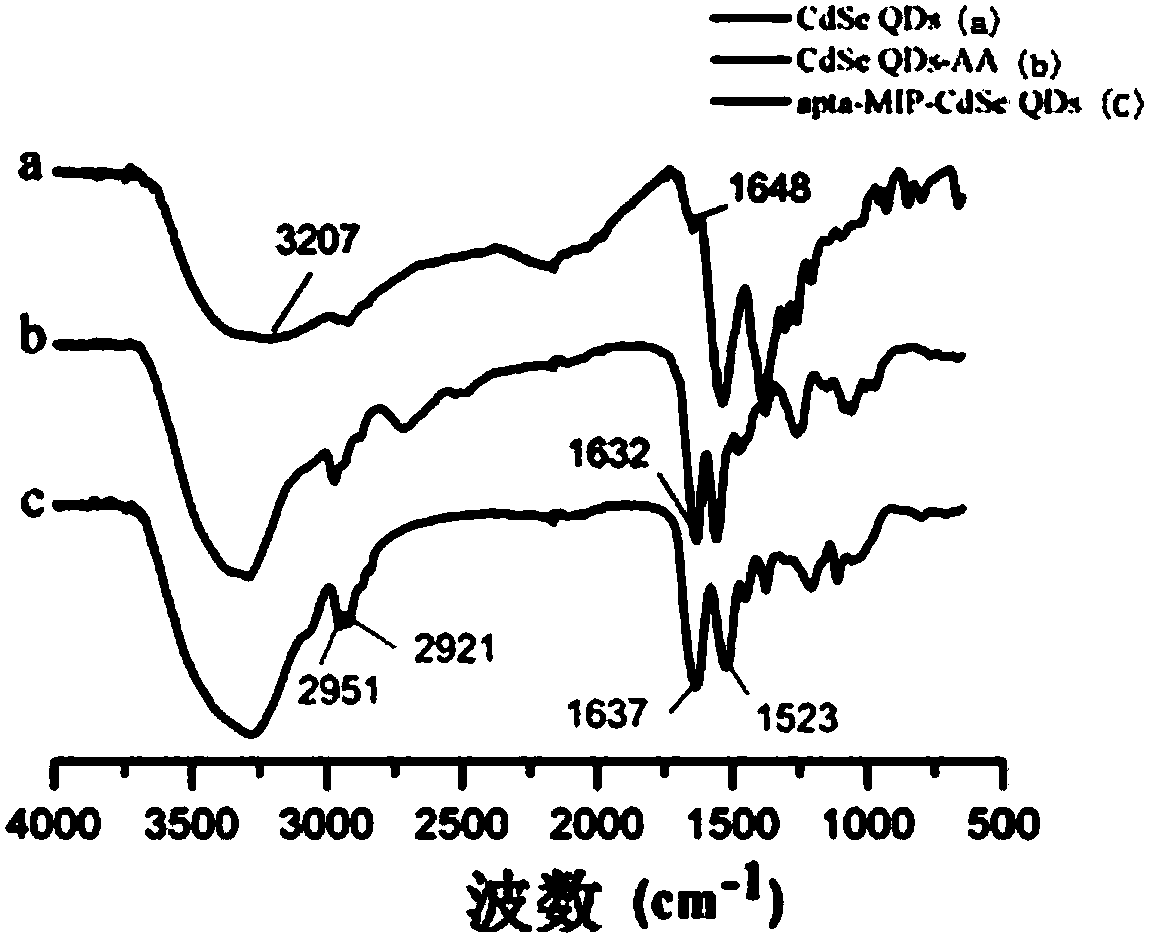
[0065] Various test in...
Embodiment 1
[0069] (1) Synthesis of carboxyl-modified CdSe quantum dots (CdSe QDs)
[0070] A: Weigh 2.3g of selenium powder and 2.3g of sodium borohydride with an analytical balance, stir slowly under a nitrogen atmosphere to dissolve them in 25ml of ultrapure water and react. NaHSe precursor solution.
[0071] B: Weigh 0.228g 2.5 chromium chloride (CrCl 2 2.5H 2 (2), join in the three-neck round-bottomed flask of 250ml, add the ultrapure water of 100ml and make it dissolve, then slowly add 97.5 microliters of 3-mercaptopropionic acid while stirring, then add freshly prepared 1M sodium hydroxide dropwise The solution adjusted the pH of the solution to 11.
[0072] C: Nitrogen was passed for half an hour to remove the dissolved oxygen in the solution, and then the freshly prepared NaHSe precursor solution was quickly added to the mixture under vigorous magnetic stirring, and then refluxed at 100°C for an appropriate time to obtain orange transparent CdSe QDs solution.
[0073] D: Add...
Embodiment 2
[0086] (1) Synthesis of carboxyl-modified CdSe quantum dots (CdSe QDs)
[0087] The synthesis method of the above carboxyl-modified CdSe quantum dots (CdSe QDs) in this example is the same as that in Example 1.
[0088] (2) Synthesis of allylamine-modified CdSe quantum dots (CdSe QDs-AA)
[0089] The synthesis method of the above-mentioned allylamine-modified CdSe quantum dots (CdSe QDs-AA) in this example is the same as that in Example 1.
[0090] (3) Preparation of an aptamer-molecularly imprinted fluorescent sensor (apta-MIP-CdSe QDs) with dual specific recognition for kanamycin
[0091] 1) Preparation of pre-polymerized complex
[0092] Weigh 1 mg of kanamycin with a balance, dissolve it in 1 ml of phosphate buffer solution with a pH value of 7, and prepare a 1 mg / ml storage solution. Dissolve 2.8nmol of kanamycin, 2.8nmol of thiol-modified kanamycin aptamer and 5.6nmol of methacrylic acid (MAA) in 0.1ml of phosphate buffer, mix well and place in a 37°C water bath Cond...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com