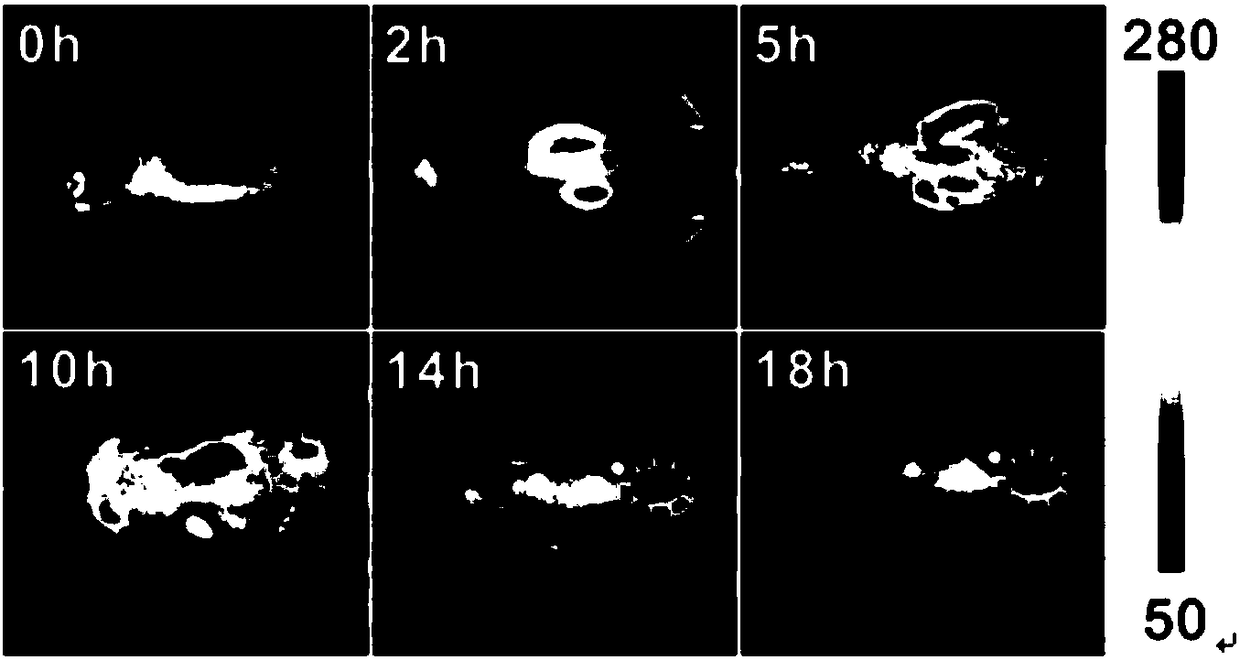
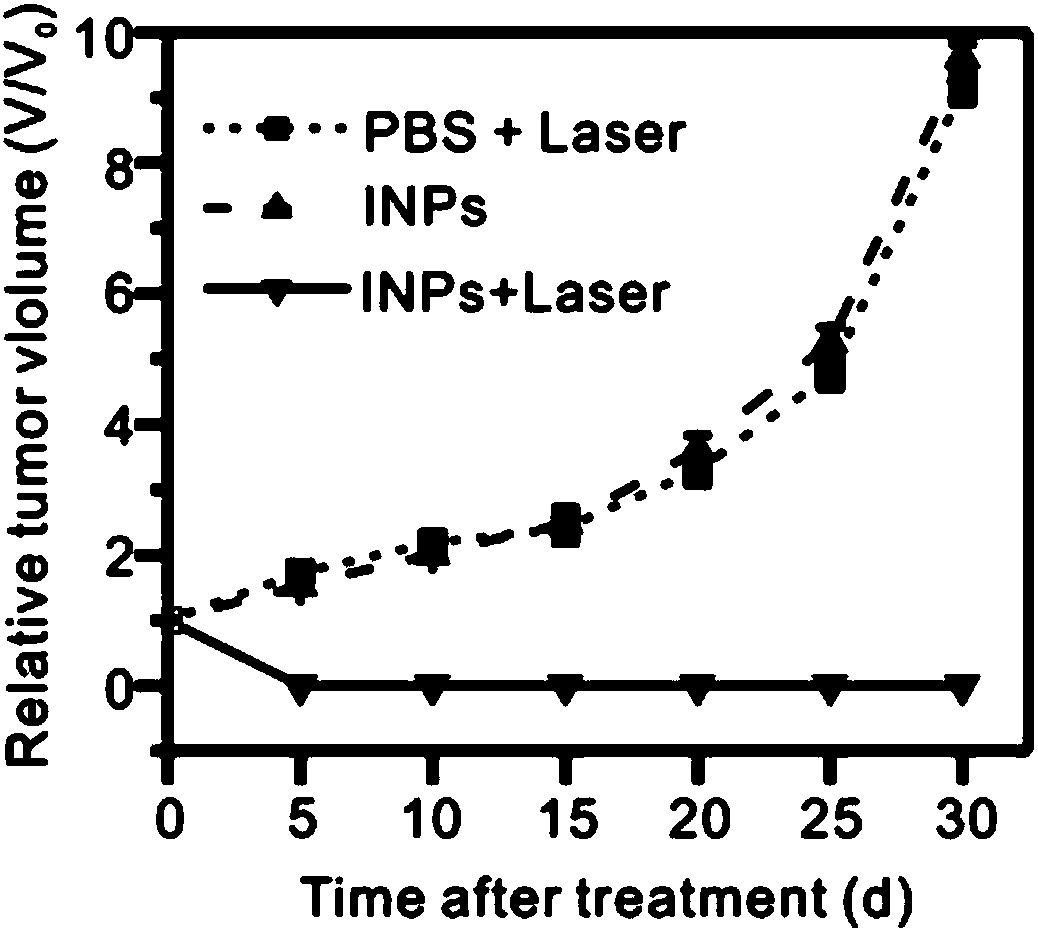
Indocyanine green derivative and preparaiton method
A technology of indocyanine green and derivatives, which is applied in the field of indole derivatives, can solve the problems of easy leakage of ICG and unstable nanoparticle structure, and achieve the effect of expanding the scope of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of Compound 3
[0035] Add compound 1 (345.0g, 1.0mol) and compound 2 (313.0g, 1.1mol) into boiling acetic anhydride (2L) under stirring conditions, stir for 30min, cool to room temperature, filter with suction, filter cake with acetone After washing and drying, a blue-black solid was obtained, which was Compound 3 (460.7 g, 85%).
Embodiment 2
[0037] Synthesis of compound 6
[0038] Compound 4 (5.7g, 27.2mmol) and compound 5 (6.90g, 35.4mmol) were dissolved in 240ml of acetonitrile and heated at 80°C for 16 hours. The reaction solution was concentrated under reduced pressure, 500ml of ether was added, and then filtered, and the residue was After washing with ether, the filter cake was dried to obtain compound 6 (9.8 g, 74.4%).
Embodiment 3
[0040] Synthesis of compound 7
[0041] Compound 3 (300mg, 0.553mmol) and compound 6 (179mg, 0.553mmol) were dissolved in 5ml of pyridine, stirred at 50°C for 1 hour; the reaction mixture was concentrated under reduced pressure, and the residue was dissolved in 10ml of water, and the solution was After the pH value was adjusted to 3, the solvent was removed by rotary evaporation under reduced pressure, and the eluent was passed through a silica gel column with chloroform / methanol (10:1) to obtain a dark green solid, which was compound 7 (105 mg, 25.9%).
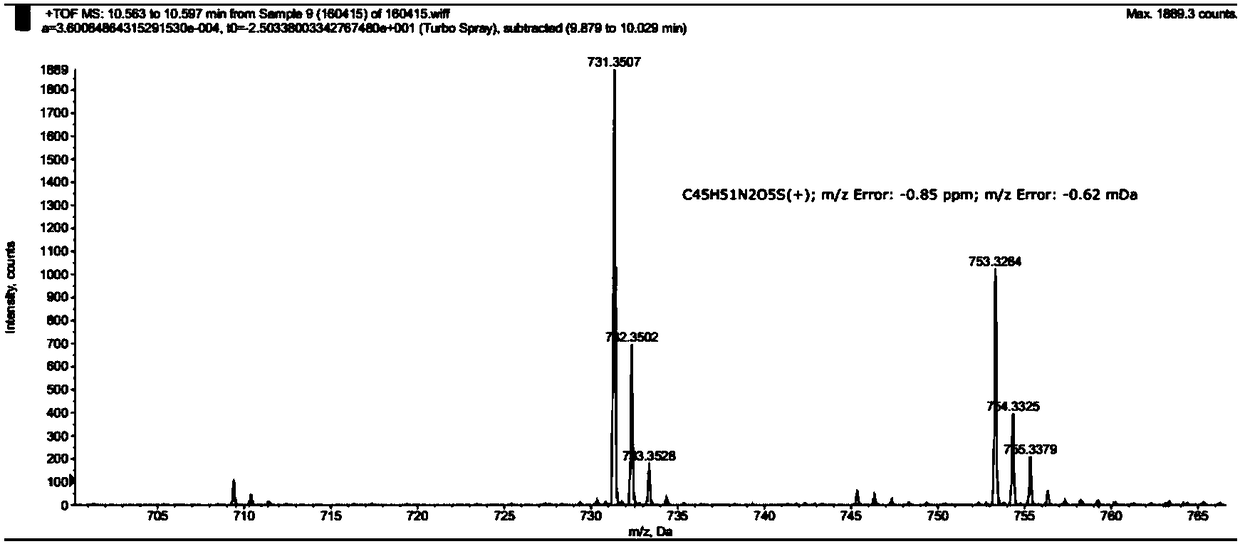
[0042] The mass spectrum data of the synthesized compound 7 is as follows: HRMS (ESI) [M+H] 731.3507, [M+Na] 753.3264, the spectrum is as follows figure 1 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com