Anti-fibrosis medicine nanometer preparation and preparation method thereof
An anti-fibrosis, nano-preparation technology, applied in drug combinations, pharmaceutical formulations, digestive system, etc., can solve the problems of targeted absorption of nano-preparations, failure to be tried and verified, affecting drug treatment effects, etc., to achieve high-efficiency anti-hepatic Fibrotic drug delivery, efficient liver fibrosis treatment, and the effect of improving availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
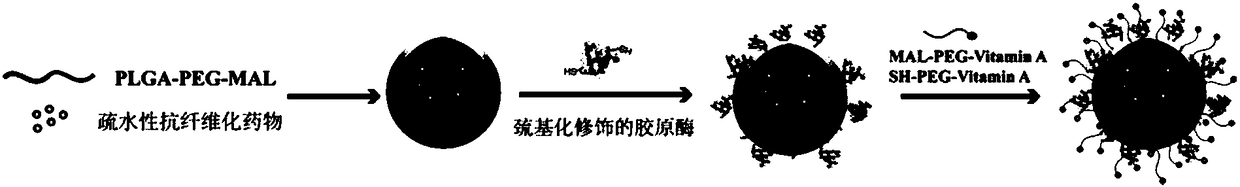
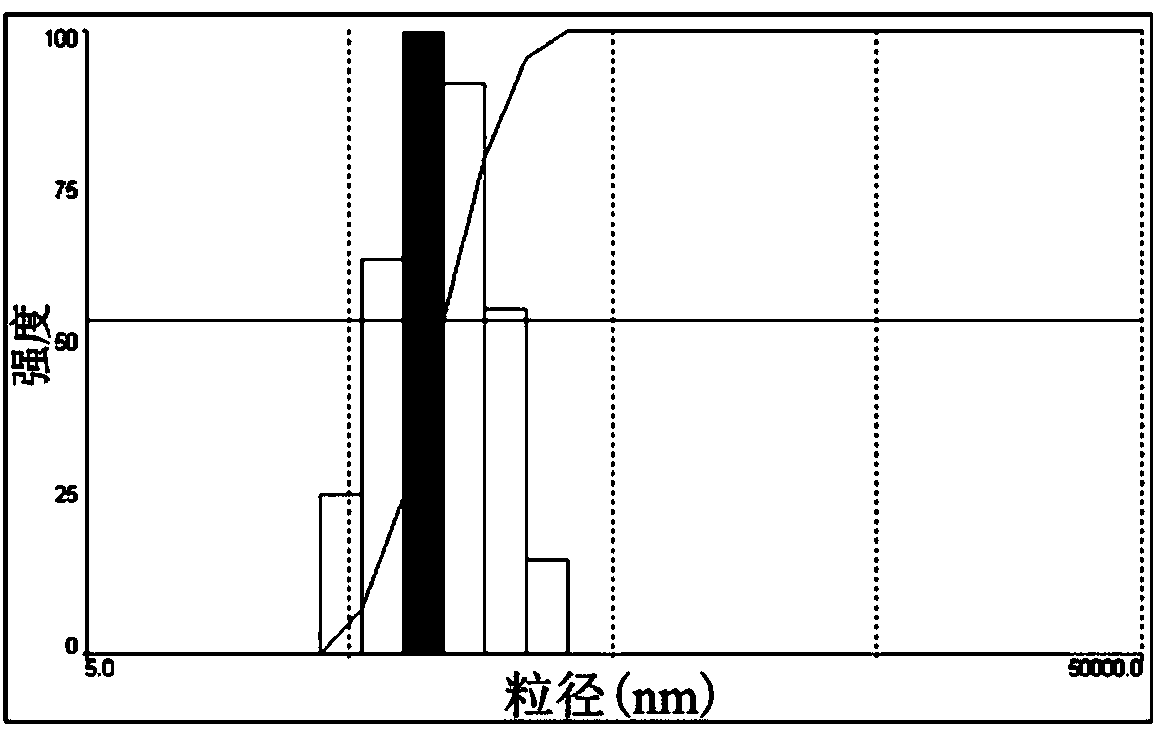
[0051] Embodiment 1 Synthesis and preparation of nano-preparation components, such as figure 1 Shown:
[0052] 1. Synthesis of MAL-PEG-VA and SH-PEG-VA
[0053] Weigh 100 mg of MAL-PEG-NHS or SH-PEG-NHS and dissolve in 5 mL of DMSO solution, weigh 22.5 mg of vitamin A and dissolve in the above solution, under dark conditions, stir and react overnight, dialyze out the DMSO solution, The unreacted vitamin A was removed by micromembrane filtration, and then freeze-dried for later use.
[0054] 2. Thiolated Collagenase I
[0055] Weigh 100 mg type I collagenase and 2.75 mg 2-iminothiolane hydrochloride, dissolve them in 5 mL 0.01M phosphate buffer, react at room temperature for 1 h, and place on a Sephadex column (G -25) Desalting and purification, UV (280 nm) detection of protein concentration in the purified solution, set aside.
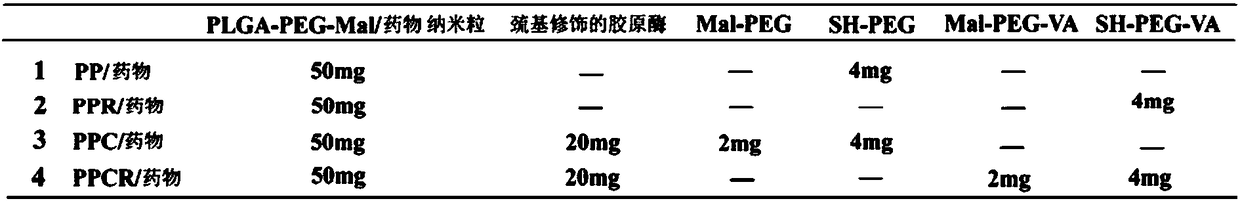
[0056] 3. Preparation of drug-loaded PLGA-PEG-MAL nanoparticles
[0057] Polymer nanoparticles loaded with anti-fibrotic drugs or fluorescent dye...
Embodiment 2
[0062] Example 2 The investigation of the safety of nano preparation carrier material
[0063] The cytotoxicity of PP, PPR, PPC and PPCR empty vectors to various liver cells (L02, LX2 and HSC-T6) was determined by MTT method. The specific operation steps are as follows: first, the cells in the logarithmic growth phase were added to 96-well plates , the plating density per well is 1×10 4 / 200 μL, cultured in a cell culture incubator for 24 h. Then use FBS-free medium to dilute the PP, PPR, PPC and PPCR empty vector groups into a series of concentration gradients, and use the cells that are not given materials as the control group, and the cells that only add medium to the blank wells as the blank group. The drug concentrations administered to the cells were 5, 10, 20, 50, 100 μg / mL, respectively. Aspirate the medium in the 96-well plate, then add 100 μL of samples with different concentration gradients to each well, and repeat 5 wells for each concentration. Continue to cu...
Embodiment 3
[0065] Example 3 Quantitative analysis of uptake of coated coumarin 6 nanometer preparation by flow cytometry in LX2 and L02 cells
[0066] Nano-preparations of PP / coumarin 6, PPR / coumarin 6, PPC / coumarin 6 and PPCR / coumarin 6 were prepared as described in Example 1. The LX2 and L02 cells were divided into 1 × 10 5 / 1mL inoculated in a 24-well plate, grown adherently in a 5% CO2 cell culture incubator at 37 °C for 24 h, sucked off the medium, and added 500 μL of free coumarin 6, PP / coumarin 6, PPR / coumarin 6, PPC / coumarin 6, and PPCR / coumarin 6 serum-free medium solutions, each group was provided with three auxiliary wells, and the concentration of coumarin 6 contained was 0.025 mg / mL Uniform standards. Continue to culture for 6 hours, wash with PBS three times, digest with trypsin, centrifuge at 2000 rpm to pellet the cells, resuspend the cells in serum-free medium, and quantitatively detect the uptake of coumarin 6 by flow cytometry.
[0067] The cell uptake data measu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com