Fluorescent probe for detecting formaldehyde-targeted mitochondria as well as preparation method and application thereof
A fluorescent probe, mitochondrial technology, used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problem of unclear content fluctuation, achieve excellent performance and reduce light damage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Synthesis of Fluorescent Probes
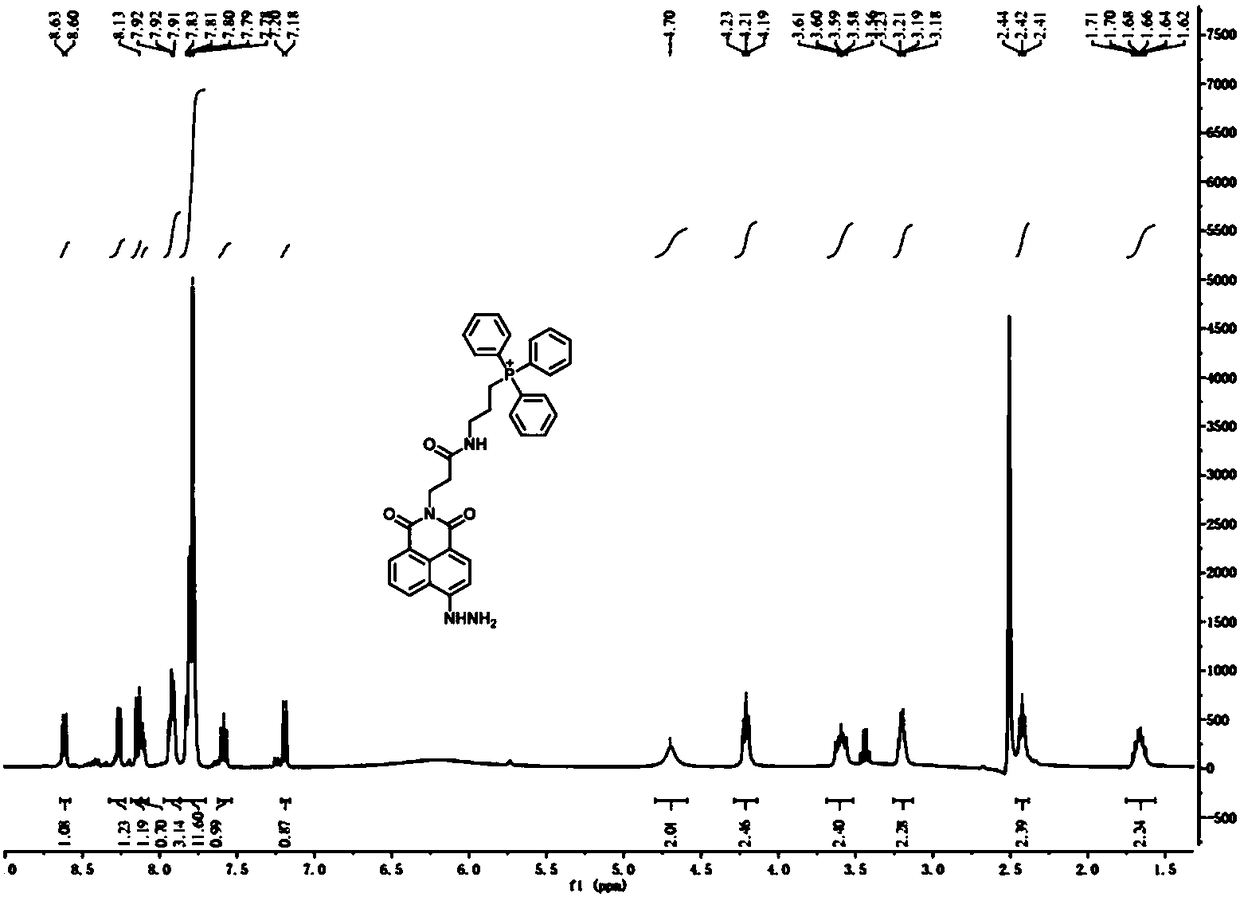
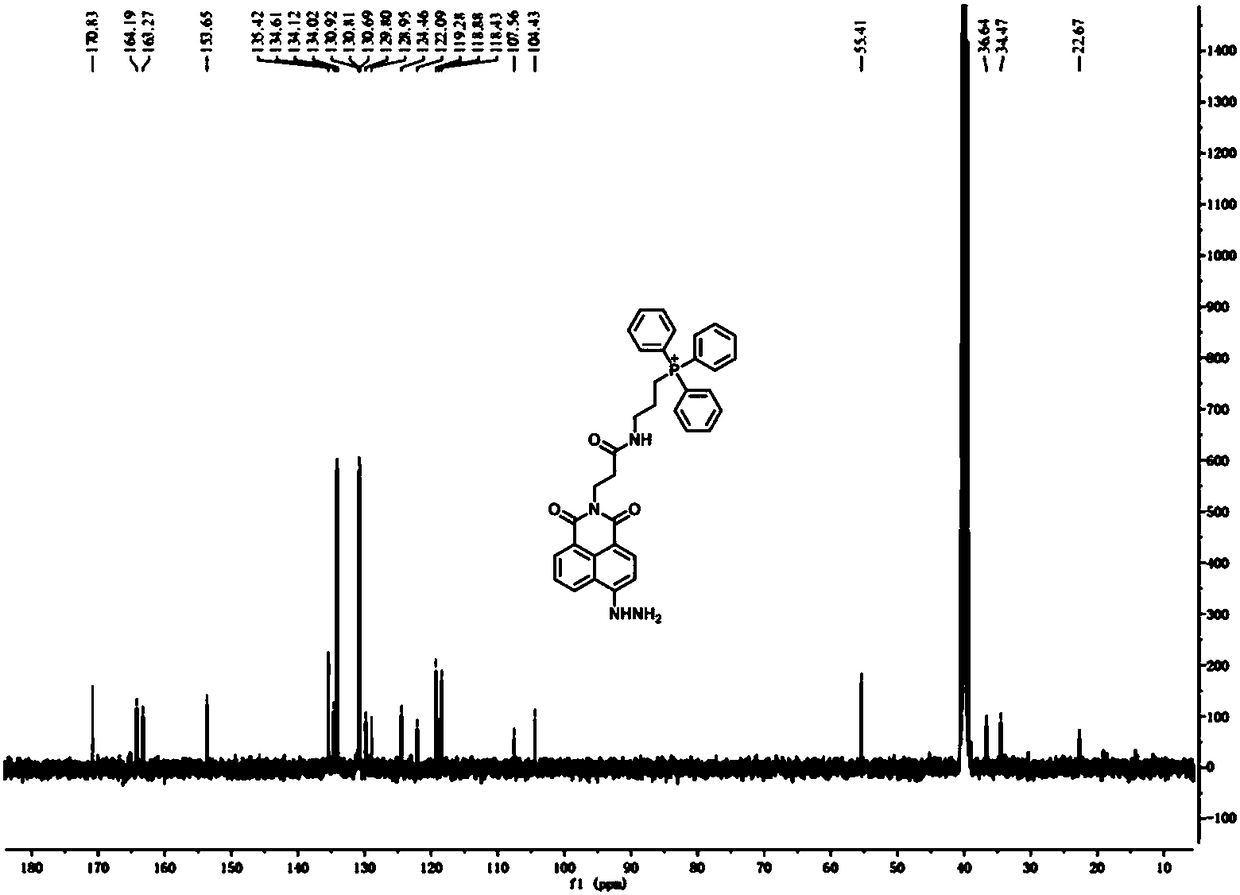
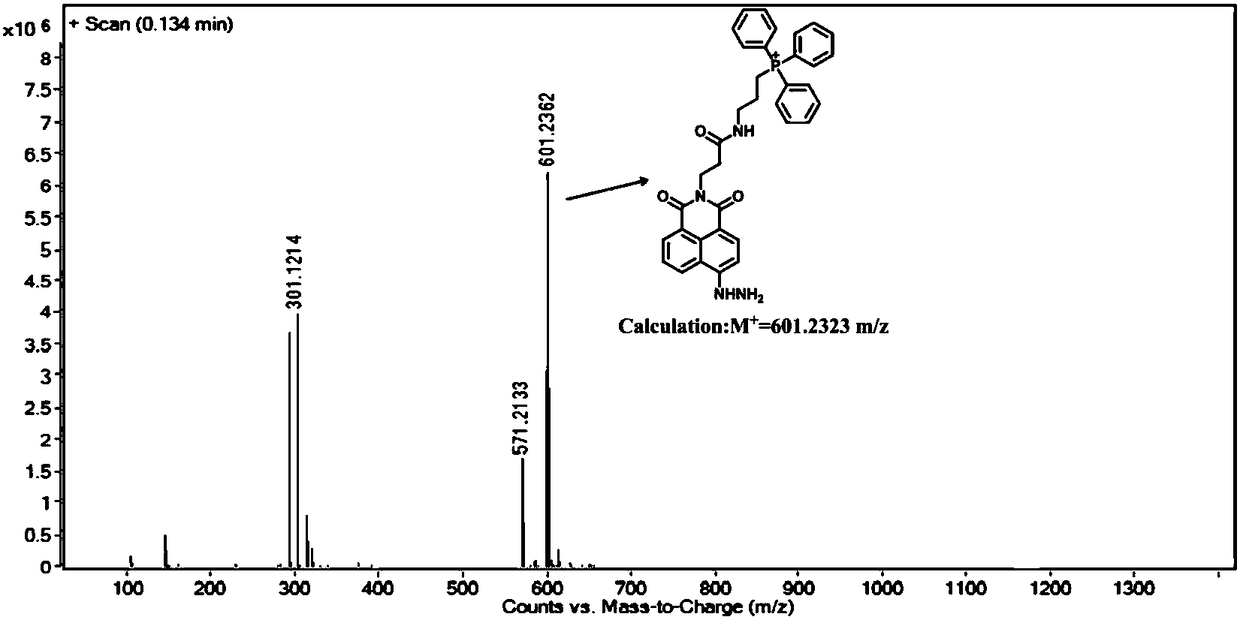
[0040] (1) Synthesis of compound 3-aminopropyltriphenylphosphonium: In a 100 mL round bottom flask, add 5 mmol of 3-bromopropylamine and 6 mmol of triphenylphosphine, then add 30 mL of acetonitrile, and heat under reflux for 24 h , cooled to room temperature, evaporated to dryness under reduced pressure, separated by silica gel column (DCM:CH 3 OH=10:1), dried in vacuo to obtain 3-aminopropyltriphenylphosphonium (1) as a white solid. Yield: 60%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 7.97 – 7.76 (m, 15H), 3.83 – 3.68 (m, 2H), 2.99 (t, J = 6 Hz, 2H), 1.84 (m, 2H); 13 C NMR (101 MHz, DMSO- d 6 ) δ 135.59, 135.56, 134.15, 134.04, 130.91, 130.79, 118.96, 118.11, 20.58, 19.11, 18.58.
[0041] (2) Synthesis of compound N-(2-formylethyl)-4-bromo-1,8-naphthalimide: In a 100 mL round bottom flask, compound 4-bromo-1,8 -5 mmol of naphthalic anhydride was added to the β - Reflux and stir in the ethanol solution of aminopropionic acid...
Embodiment 2
[0044] Example 2 Changes in the fluorescence intensity of Na-FA-Mito under different concentrations of formaldehyde
[0045] Prepare 10 mL of aqueous solutions of 100 mM, 10 mM, 1 mM, and 0.1 mM formaldehyde and the fluorescent probe mother solution prepared in Example 1 at a concentration of 1 mM as spares.
[0046] The PBS buffer solution (pH=7.4, 5% DMSO) with a probe concentration of 5 μM was added to interact with different concentrations of formaldehyde (0 – 250 μM), and the fluorescence spectrum was tested after one hour of action, (λex = 440 nm , λem =450-700 nm), detect the fluorescence intensity in each system, and use the fluorescence intensity-formaldehyde concentration to draw a curve, such as Figure 4 As shown in the middle and large picture, the curve is drawn from the 539nm fluorescence intensity-formaldehyde concentration, such as Figure 4 As shown in the middle and small panels: As the concentration of formaldehyde increases, the fluorescence intensity of ...
Embodiment 3
[0047] Example 3 Changes in the fluorescence intensity of Na-FA-Mito under different reaction times
[0048] Prepare 10 mL of an aqueous solution with a concentration of 100 mM formaldehyde and the fluorescent probe mother solution prepared in Example 1 with a concentration of 1 mM as spares.
[0049] Add 150 μM formaldehyde or an equivalent amount of PBS buffer solution (pH=7.4, 5% DMSO) with a probe concentration of 5 μM, test once every 2 min, and test for 1 h. The excitation wavelength is 440 nm, whichever is the maximum Emission peak position λ em The fluorescence intensity at =539nm draws its kinetic curve with time, such as Figure 5 As shown, the fluorescence intensity of the probe did not change significantly when irradiated in PBS buffer solution (pH=7.4, 5% DMSO) for 1 h; however, the kinetic experiment of the probe with formaldehyde (150 μM) showed that the fluorescence intensity was about It tends to saturation at 40 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com