Excoecaria agallocha endophytic fungus sourced indene derivative, and applications thereof in preparation of anti-inflammatory drugs
A technology of endophytic fungi and derivatives, applied in the field of pharmaceutical compounds, can solve problems such as injury, adverse reactions, kidney damage, etc., and achieve good results in application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The acquisition of embodiment 1 sea paint endophytic fungus Diaporthe sp.SYSU-HQ3
[0042] The inventor team isolated an endophytic fungus Diaporthe sp.SYSU-HQ3 from the fresh leaves of the mangrove plant Excoecaria agallocha L. in the sea area of Zhuhai, Guangdong Province, and preserved it in Guangdong Microbial Bacteria on August 4, 2017. Species Collection Center (GDMCC), the deposit number is GDMCC No: 60217.
Embodiment 2
[0043] The separation of embodiment 2 compound
[0044] Novel indene derivatives were isolated from the fermentation broth of endophytic fungus Diaporthe sp.SYSU-HQ3.
[0045] The specific method is as follows:
[0046] (1) Seed liquid culture of the fungus Diaporthe sp.SYSU-HQ3: The composition of the medium is by weight: 0.3% glucose, 0.1% yeast extract, 0.5% peptone, 2.5% agar, 3% sodium chloride, and 98% water ; Make a test tube slant, pick the strains into the slant, and culture at 30°C for 6 days.
[0047] (2) Fermentation culture of the fungus Diaporthe sp.SYSU-HQ3: use solid rice fermentation medium: rice: seawater = 1:1; moon;
[0048] (3) Extracting the product after the above-mentioned fermentation culture with methanol three times, concentrating the extract, and extracting the obtained concentrated extract with ethyl acetate to obtain a crude ethyl acetate extract.
[0049] (4) The ethyl acetate crude extract is separated by silica gel normal phase chromatograp...
Embodiment 3
[0050] The structural analysis of embodiment 3 compound
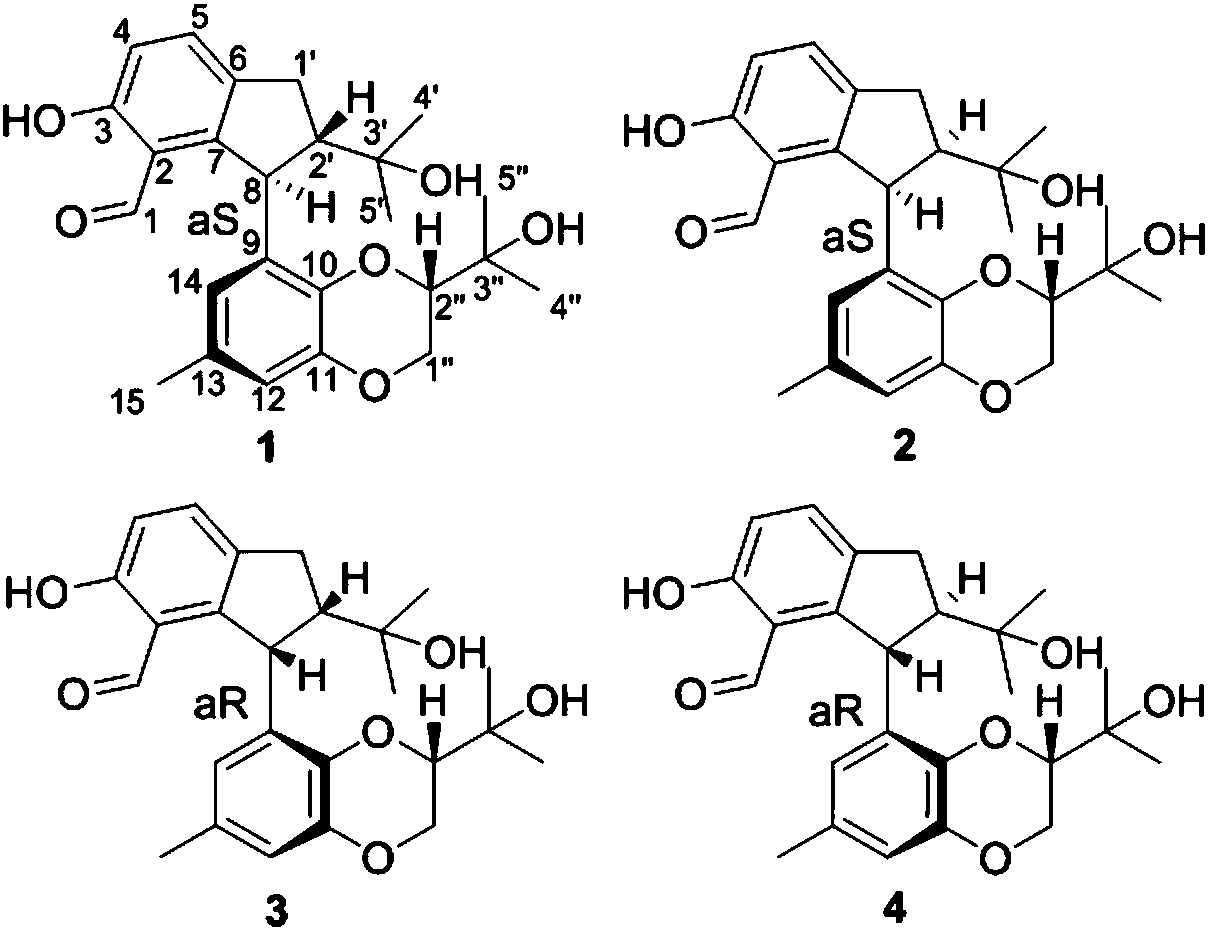
[0051] Structural analysis of new compounds 1-4 was carried out, and the following experimental data were obtained:
[0052] New Compound 1: C 25 h 30 o 6 , HRESI-MS: 425.1961 [M-H] - (calculated value 425.1964).
[0053] New compound 2: C 25 h 30 o6 , HRESI-MS: 425.1968 [M-H] - (calculated value 425.1964).
[0054] New Compound 3: C 25 h 30 o 6 , HRESI-MS: 425.1964 [M-H] - (calculated value 425.1964).
[0055] New Compound 4: C 25 h 30 o 6 , HRESI-MS: 425.1968 [M-H] - (calculated value 425.1964).
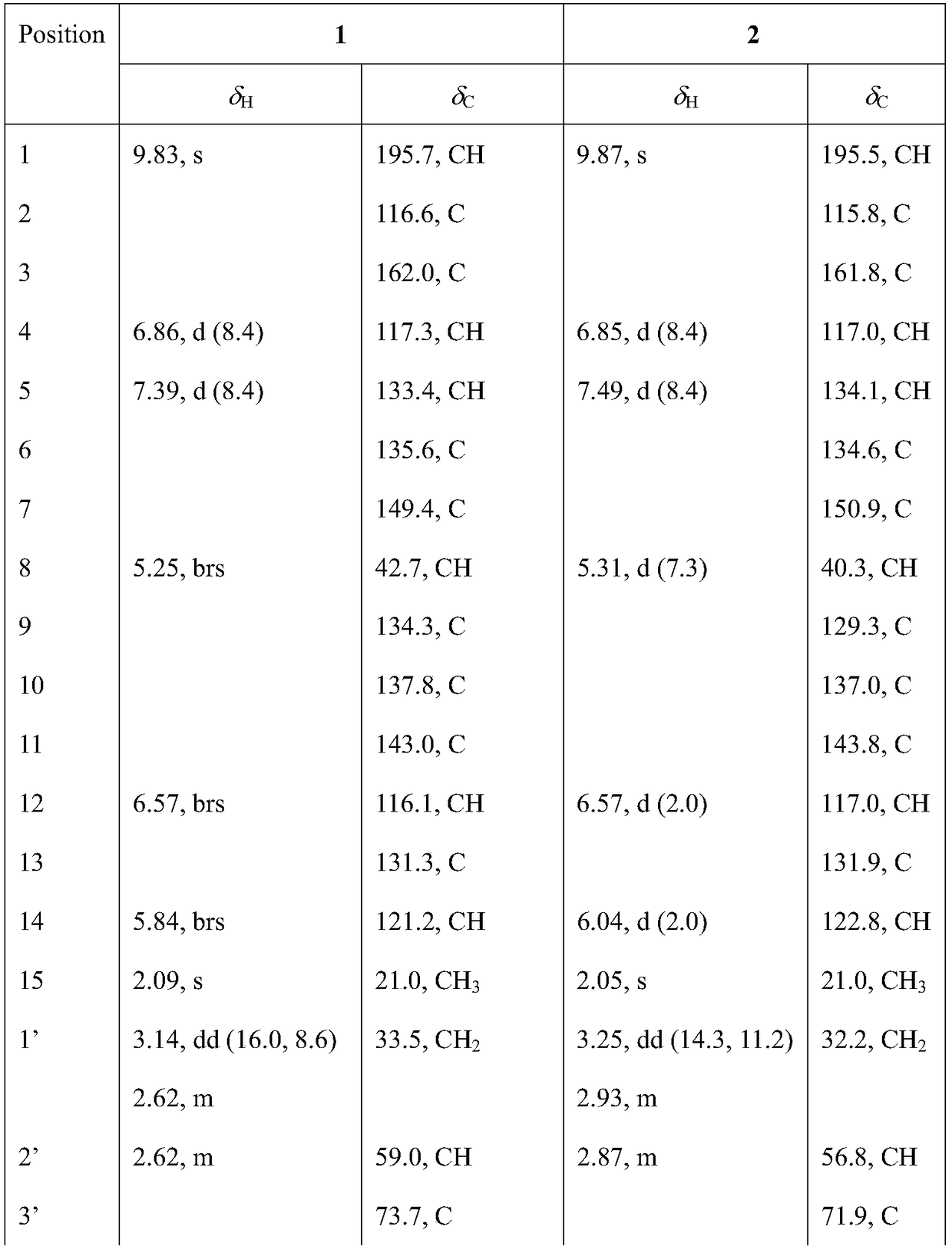
[0056] The NMR data of compounds 1-4 are shown in Table 1 and Table 2.
[0057] Table 1. NMR data of compounds 1 and 2 (CDCl 3 , 500MHz / 125MHz,ppm)
[0058]
[0059]
[0060] Table 2. NMR data of compounds 3 and 4 (CDCl 3 , 500MHz / 125MHz,ppm)
[0061]
[0062]
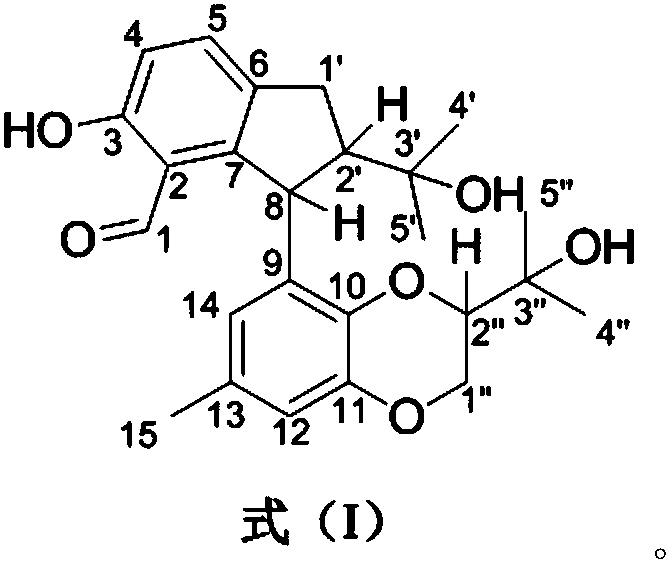
[0063] The structural formulas of compounds 1-4 are as follows:
[0064]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com